The potassium iodate formula is written as KIO3. potassium iodate is made by reacting potassium bases, such as KOH, with iodic acid. It is one of the most often used compounds for iodizing table salt. Potassium iodate is also used to precipitate thorium, particularly to separate it from rare earth elements. It is mostly employed as an oxidizing agent in practical chemistry. Potassium iodate is extremely dangerous since it can rapidly catch fire if it comes into touch with reducing agents.
Potassium Iodate Formula
Potassium Iodate (KIO3) is an ionic compound composed of potassium cation (K+) and iodate anion (IO3-). In kl solution, KIO3 is soluble. Alcohol, liquid ammonia, and nitric acid are all insoluble in KIO3. KIO3 has an atomic mass of 39.0983, iodine has an atomic mass of 126.90447, and oxygen has an atomic mass of 15.9993. Potassium iodate is an oxidizing chemical that can cause flames when comes into contact with combustible or reducing materials. Iodine is necessary for optimum health and is required for the thyroid gland to operate properly, which influences the body’s metabolic and immune activities. Radiation exposure is specified as a ‘thyroid blocking agent’ to inhibit radioactive iodine uptake.
KIO3 Chemical Name
The chemical name of KIO₃ is Potassium Iodate. It is an inorganic compound consisting of potassium (K⁺) and iodate (IO₃⁻) ions. Potassium iodate is often used in iodized salt to prevent iodine deficiency.
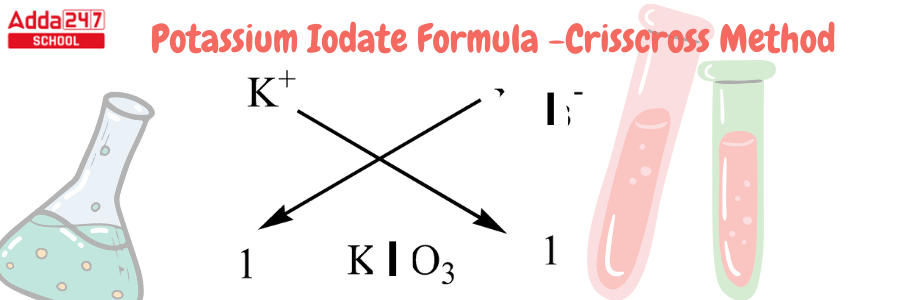
Potassium Iodate Formula by Criss Cross Method
Criss Cross Method – All chemical formulas can be written in this crisis cross manner. The numerical value of the ion charge between the two atoms is crossed across in the criss-cross method, which forms the subscript of the other ion. Using this method, we will develop the chemical formula for potassium iodide.
Development of Potassium Formula using Criss Cross Method-
- We must recall that Potassium has an atomic number of 19 and a valency of 1. It has one electron for bonding in the outermost shell.
- Iodine has an atomic number of 53 and 1 electron in its outermost shell. Iodides are iodine compounds with an oxidation state of -1. Iodide is an anion with a valency of -1.
- because it lacks one atom to complete its octet. As a result, we only require one molecule of iodide for one atom of potassium.
- For this compound, we can use the criss-cross approach as follows:
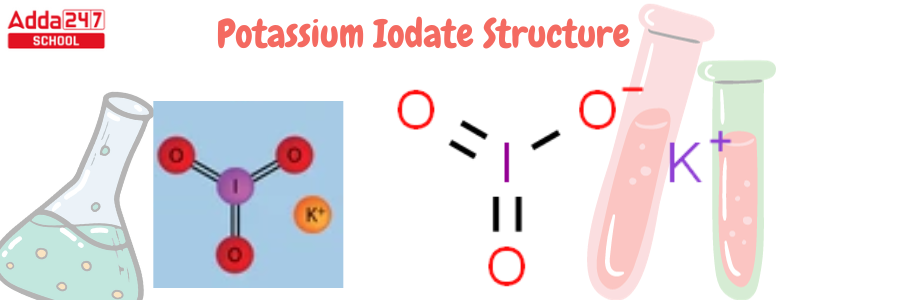
Potassium Iodate Structure
KIO3 is the potassium iodate formula. The chemical formula KIO3, with a molar mass of 214.001 g/mol, describes the structure of potassium iodate. It is made up of K+ ions and IO3- ions, which include potassium, iodide, and oxygen. The following is the structure of potassium iodate.
Potassium Iodate (KIO3) Preparation
Iodic acid reacts with a potassium-containing base, generally potassium hydroxide, to yield potassium iodate, which is one of the simplest ways to make the compound. The following is the reaction:
HIO3 + KOH → KIO3 + H2O
In-laboratory preparation of 0.05 M Potassium Iodate
The next section discusses the preparation and standardization of 0.05 M potassium iodate employing Sodium Thiosulphate and Starch solution as indicators.
Preparation of Potassium Iodate Solution –
Weigh precisely 10.7 g of potassium iodate, dried to constant weight at 110°C, in 1000 ml of water.
Standardize the solution by doing the following –
- Standardization of Potassium Iodate Solution
- Dilute 25.0 mL of the solution with water to make 100 mL.
- Add 2 g potassium iodide and 10 ml 1M sulphuric acid to 20.0 ml of this solution.
- Titrate with 0.1 M sodium thiosulphate, using 1 ml of starch solution as an indicator near the conclusion of the titration.
- 1 ml of 0.1 M sodium thiosulphate equals 0.003566 g KIO3.
Physical Properties of Potassium Iodate
The physical properties of Potassium iodide are tabulated below.
| Potassium Iodate Physical Properties | |
| Color | White |
| Odor | Odorless |
| Appearance |
White crystalline powder
|
| Covalently-Bonded Unit | 2 |
| Solubility |
Soluble in KI solution, moderately soluble in cold water, solubility increases in boiling water, insoluble in alcohol.
|
| Heat Capacity |
313 J Kg-1 K-1
|
| Melting Point | 560º C |
| Boiling Point | 100ºC |
| Hydrogen Bond Acceptor | 3 |
| Complexity | 49.8 |
| Density | 3.90 g/cm3 |
| Molar Mass | 214.001 g/mol |
Potassium Iodate Chemical Properties
The chemical properties of potassium iodate are detailed here.
- When strong acids like sulfuric acid are present, potassium iodate and potassium iodide mix to generate potassium sulphate, iodine, and water.
KIO3 + 5KI + 3H2SO4 → 3K2SO4 + 3H2O + 3I2 - When potassium iodide is titrated with chlorinated water in a neutral solution, the reaction that occurs is described by the equation –
Kl + 3Cl2 + 3H2O → KCl + HIO2 + 5HCl
- Silver iodate and potassium nitrate are formed when potassium iodate interacts with silver nitrate. The chemical reaction is illustrated below.
KIO3 + AgNO3 → AgIO3 + KNO3 - However, it is not widely known if a high oversupply of free hydrochloric acid is present throughout the titration, and chloroform or carbon tetrachloride is employed as an indicator. the reaction will be –
Kl + Cl2 → KCl + ICl
Potassium Iodate Formula and Uses
Potassium iodate has the following applications.
- Iodate is utilized as a source of iodine in the diet because it oxidises to iodine in an atmosphere of oxygen in damp circumstances.
- In cuisine, it is used as a maturing agent and dough conditioner.
- They are used as reagents and additives to feed livestock.
- It is employed in iodometric analytical procedures to detect the presence of zinc and arsenic.
- It is used in the medical field to prevent radiation illness.
Potassium Iodine Disulphate Formula
The chemical formula for potassium iodine disulfate is KIO(SO₄)₂. It is a compound that consists of potassium (K), iodine (I), and disulfate groups (SO₄).
Potassium Iodide Disulphide Formula
The chemical formula for potassium iodide disulfide is KI.S₂ or KI₂S₂, depending on the specific form of the compound.
- KI.S₂ refers to a mixture or complex where potassium iodide (KI) is combined with disulfur (S₂).
- KI₂S₂ could represent a specific stoichiometric compound, though it is less common.
In standard cases, potassium iodide and sulfur can interact, but there isn’t a widely recognized single compound known strictly as potassium iodide disulfide.
100 Chemical Formula by Criss-Cross Method
The criss-cross method is used to determine the chemical formula of ionic compounds. It involves swapping the charges (oxidation states) of the cation (positive ion) and anion (negative ion) to balance out the charges and make a neutral compound. Below are 100 chemical formulas derived using the criss-cross method:
1-25
Sodium chloride (NaCl)
Na^+ + Cl^- → NaCl
Magnesium oxide (MgO)
Mg^2+ + O^2- → MgO
Calcium fluoride (CaF₂)
Ca^2+ + F^- → CaF₂
Aluminum oxide (Al₂O₃)
Al^3+ + O^2- → Al₂O₃
Potassium bromide (KBr)
K^+ + Br^- → KBr
Barium sulfate (BaSO₄)
Ba^2+ + SO₄^2- → BaSO₄
Iron (III) chloride (FeCl₃)
Fe^3+ + Cl^- → FeCl₃
Zinc sulfate (ZnSO₄)
Zn^2+ + SO₄^2- → ZnSO₄
Copper (II) oxide (CuO)
Cu^2+ + O^2- → CuO
Sodium nitrate (NaNO₃)
Na^+ + NO₃^- → NaNO₃
Calcium phosphate (Ca₃(PO₄)₂)
Ca^2+ + PO₄^3- → Ca₃(PO₄)₂
Ammonium chloride (NH₄Cl)
NH₄^+ + Cl^- → NH₄Cl
Lithium oxide (Li₂O)
Li^+ + O^2- → Li₂O
Aluminum sulfide (Al₂S₃)
Al^3+ + S^2- → Al₂S₃
Magnesium nitrate (Mg(NO₃)₂)
Mg^2+ + NO₃^- → Mg(NO₃)₂
Potassium sulfate (K₂SO₄)
K^+ + SO₄^2- → K₂SO₄
Iron (II) oxide (FeO)
Fe^2+ + O^2- → FeO
Calcium hydroxide (Ca(OH)₂)
Ca^2+ + OH^- → Ca(OH)₂
Sodium carbonate (Na₂CO₃)
Na^+ + CO₃^2- → Na₂CO₃
Zinc chloride (ZnCl₂)
Zn^2+ + Cl^- → ZnCl₂
Aluminum nitrate (Al(NO₃)₃)
Al^3+ + NO₃^- → Al(NO₃)₃
Magnesium phosphate (Mg₃(PO₄)₂)
Mg^2+ + PO₄^3- → Mg₃(PO₄)₂
Copper (I) sulfide (Cu₂S)
Cu^+ + S^2- → Cu₂S
Potassium carbonate (K₂CO₃)
K^+ + CO₃^2- → K₂CO₃
Lead (II) chloride (PbCl₂)
Pb^2+ + Cl^- → PbCl₂
26-50
Iron (III) sulfate (Fe₂(SO₄)₃)
Fe^3+ + SO₄^2- → Fe₂(SO₄)₃
Sodium sulfate (Na₂SO₄)
Na^+ + SO₄^2- → Na₂SO₄
Magnesium bromide (MgBr₂)
Mg^2+ + Br^- → MgBr₂
Calcium carbonate (CaCO₃)
Ca^2+ + CO₃^2- → CaCO₃
Aluminum phosphate (AlPO₄)
Al^3+ + PO₄^3- → AlPO₄
Potassium nitrate (KNO₃)
K^+ + NO₃^- → KNO₃
Copper (II) sulfate (CuSO₄)
Cu^2+ + SO₄^2- → CuSO₄
Zinc carbonate (ZnCO₃)
Zn^2+ + CO₃^2- → ZnCO₃
Magnesium chloride (MgCl₂)
Mg^2+ + Cl^- → MgCl₂
Ammonium sulfate ((NH₄)₂SO₄)
NH₄^+ + SO₄^2- → (NH₄)₂SO₄
Lithium nitrate (LiNO₃)
Li^+ + NO₃^- → LiNO₃
Barium chloride (BaCl₂)
Ba^2+ + Cl^- → BaCl₂
Sodium phosphate (Na₃PO₄)
Na^+ + PO₄^3- → Na₃PO₄
Calcium sulfide (CaS)
Ca^2+ + S^2- → CaS
Iron (II) sulfate (FeSO₄)
Fe^2+ + SO₄^2- → FeSO₄
Magnesium sulfate (MgSO₄)
Mg^2+ + SO₄^2- → MgSO₄
Potassium chromate (K₂CrO₄)
K^+ + CrO₄^2- → K₂CrO₄
Copper (II) nitrate (Cu(NO₃)₂)
Cu^2+ + NO₃^- → Cu(NO₃)₂
Zinc sulfide (ZnS)
Zn^2+ + S^2- → ZnS
Ammonium nitrate (NH₄NO₃)
NH₄^+ + NO₃^- → NH₄NO₃
Sodium bicarbonate (NaHCO₃)
Na^+ + HCO₃^- → NaHCO₃
Aluminum hydroxide (Al(OH)₃)
Al^3+ + OH^- → Al(OH)₃
Potassium dichromate (K₂Cr₂O₇)
K^+ + Cr₂O₇^2- → K₂Cr₂O₇
Barium nitrate (Ba(NO₃)₂)
Ba^2+ + NO₃^- → Ba(NO₃)₂
Calcium chloride (CaCl₂)
Ca^2+ + Cl^- → CaCl₂
51-75
Magnesium oxide (MgO)
Mg^2+ + O^2- → MgO
Iron (III) nitrate (Fe(NO₃)₃)
Fe^3+ + NO₃^- → Fe(NO₃)₃
Zinc nitrate (Zn(NO₃)₂)
Zn^2+ + NO₃^- → Zn(NO₃)₂
Sodium iodide (NaI)
Na^+ + I^- → NaI
Barium carbonate (BaCO₃)
Ba^2+ + CO₃^2- → BaCO₃
Magnesium fluoride (MgF₂)
Mg^2+ + F^- → MgF₂
Lithium bromide (LiBr)
Li^+ + Br^- → LiBr
Aluminum chloride (AlCl₃)
Al^3+ + Cl^- → AlCl₃
Copper (I) oxide (Cu₂O)
Cu^+ + O^2- → Cu₂O
Potassium hydroxide (KOH)
K^+ + OH^- → KOH
Zinc phosphate (Zn₃(PO₄)₂)
Zn^2+ + PO₄^3- → Zn₃(PO₄)₂
Ammonium phosphate ((NH₄)₃PO₄)
NH₄^+ + PO₄^3- → (NH₄)₃PO₄
Calcium nitride (Ca₃N₂)
Ca^2+ + N^3- → Ca₃N₂
Iron (II) bromide (FeBr₂)
Fe^2+ + Br^- → FeBr₂
Sodium oxide (Na₂O)
Na^+ + O^2- → Na₂O
Magnesium nitride (Mg₃N₂)
Mg^2+ + N^3- → Mg₃N₂
Potassium iodide (KI)
K^+ + I^- → KI
Barium sulfate (BaSO₄)
Ba^2+ + SO₄^2- → BaSO₄
Zinc acetate (Zn(CH₃COO)₂)
Zn^2+ + CH₃COO^- → Zn(CH₃COO)₂
Lithium carbonate (Li₂CO₃)
Li^+ + CO₃^2- → Li₂CO₃
Aluminum sulfate (Al₂(SO₄)₃)
Al^3+ + SO₄^2- → Al₂(SO₄)₃
Copper (II) bromide (CuBr₂)
Cu^2+ + Br^- → CuBr₂
Magnesium hydroxide (Mg(OH)₂)
Mg^2+ + OH^- → Mg(OH)₂
Sodium permanganate (NaMnO₄)
Na^+ + MnO₄^- → NaMnO₄
Barium fluoride (BaF₂)
Ba^2+ + F^- → BaF₂
76-100
Lithium phosphate (Li₃PO₄)
Li^+ + PO₄^3- → Li₃PO₄
Potassium permanganate (KMnO₄)
K^+ + MnO₄^- → KMnO₄
Copper (II) carbonate (CuCO₃)
Cu^2+ + CO₃^2- → CuCO₃
Ammonium sulfide ((NH₄)₂S)
NH₄^+ + S^2- → (NH₄)₂S
Zinc bromide (ZnBr₂)
Zn^2+ + Br^- → ZnBr₂
Iron (III) phosphate (FePO₄)
Fe^3+ + PO₄^3- → FePO₄
Sodium acetate (CH₃COONa)
Na^+ + CH₃COO^- → CH₃COONa
Calcium chromate (CaCrO₄)
Ca^2+ + CrO₄^2- → CaCrO₄
Magnesium acetate (Mg(CH₃COO)₂)
Mg^2+ + CH₃COO^- → Mg(CH₃COO)₂
Ammonium carbonate ((NH₄)₂CO₃)
NH₄^+ + CO₃^2- → (NH₄)₂CO₃
Sodium hypochlorite (NaClO)
Na^+ + ClO^- → NaClO
Calcium cyanide (Ca(CN)₂)
Ca^2+ + CN^- → Ca(CN)₂
Potassium oxalate (K₂C₂O₄)
K^+ + C₂O₄^2- → K₂C₂O₄
Copper (I) iodide (CuI)
Cu^+ + I^- → CuI
Lithium sulfate (Li₂SO₄)
Li^+ + SO₄^2- → Li₂SO₄
Barium hydroxide (Ba(OH)₂)
Ba^2+ + OH^- → Ba(OH)₂
Iron (II) phosphate (Fe₃(PO₄)₂)
Fe^2+ + PO₄^3- → Fe₃(PO₄)₂
Magnesium permanganate (Mg(MnO₄)₂)
Mg^2+ + MnO₄^- → Mg(MnO₄)₂
Aluminum chromate (Al₂(CrO₄)₃)
Al^3+ + CrO₄^2- → Al₂(CrO₄)₃
Sodium chromate (Na₂CrO₄)
Na^+ + CrO₄^2- → Na₂CrO₄
Copper (II) acetate (Cu(CH₃COO)₂)
Cu^2+ + CH₃COO^- → Cu(CH₃COO)₂
Zinc chromate (ZnCrO₄)
Zn^2+ + CrO₄^2- → ZnCrO₄
Lithium oxide (Li₂O)
Li^+ + O^2- → Li₂O
Potassium acetate (CH₃COOK)
K^+ + CH₃COO^- → CH₃COOK
Ammonium bicarbonate (NH₄HCO₃)
NH₄^+ + HCO₃^- → NH₄HCO₃
These formulas are derived using the criss-cross method based on the charges of the ions involved.








 CBSE Date Sheet 2026 for Class 10 & ...
CBSE Date Sheet 2026 for Class 10 & ...
 CBSE Class 10 Date Sheet 2026, Check 10t...
CBSE Class 10 Date Sheet 2026, Check 10t...
 CUET History Syllabus 2026 (Updated), Do...
CUET History Syllabus 2026 (Updated), Do...














