Table of Contents
The Propanone is one the most important chemical compound which is used extensively in our daily life. The Propanone belongs to the ketone group of organic compounds. That is why, students are often asked questions on this compound in their school exams and competitive exams. The Propanone formula is very useful that defines the chemical properties of a propanone. In this article, we will learn about the Propanone Formula, its molecular weight, structure, properties and other related details in a comprehensive manner.
Propanone Formula
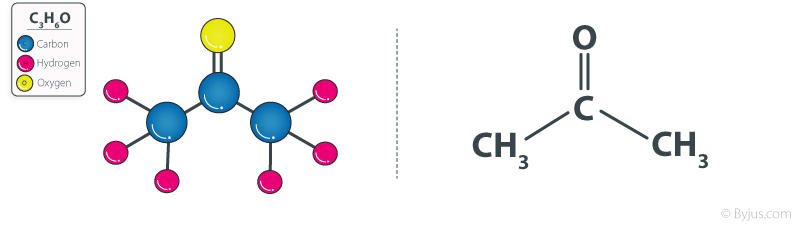
The Propane formula is C3H6O. This formula of Propanone gives it many special characteristics. Propane is also commonly known as acetone. Propane is the official IPUAC name for the Acetone. The Propanone formula, also known as acetone, is provided here in both organic and structural forms. In summary, propanone is a flammable, colorless, and volatile liquid that is the smallest and simplest Ketone. But first let us know about the propanone in brief.
What is Propanone?
Propanone is an organic compound that is extremely flammable. The chemical formula of this organic solvent is C3H6O. Its alternative name is acetone. It can be detected in the emissions of cars, factories, vegetation, and wildfires. It can also be found in the human body, typically found in urine and blood. It has no color and evaporates easily. It can dissolve in water, ether, and ethanol, with a strong, floral or irritating odor.
Propanone is commonly utilized as a disinfectant and cleaner. The first ones to create acetone were the alchemists. Metal acetates are subjected to dry distillation during the production process. At present, propanone is manufactured from propylene using either the direct or indirect method. The cumene process accounts for nearly 83% of propanone production. Furthermore, there exist additional traditional techniques for generating the propanone.
Chemical Formula of Propanone
The chemical formula of any chemical compound is important to remember as it can be used by students to deduce many important insights. The Propane chemical formula and structure is highly important to remember while defining its properties. The propane chemical formula is CH3COCH3, with a condensed formula of C3H6O.
Propanone Molecular Weight
The molecular weight of a compound in chemistry is the sum of weights of all the elements/atoms present in it. The molecular weight of Propanone is 58.08 grams per mole. In addition, it is the most basic type of ketone that is produced from a three-carbon chain where two of the hydrogen atoms on the second carbon are replaced by an oxygen atom double-bonded to a carbon atom.
Propanone Density g/ml
Density is basically the amount of mass present per volume of the compound. One can deduce the density of propanone by knowing the propanone formula. The density of propanone is 0.7845 g/cm3 (25 °C) or 0.7845 g/ml at the room temperature. It is highly important to note that density of every compound including the propanone changes with change in temperature.
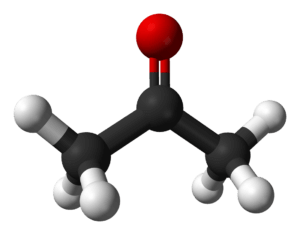
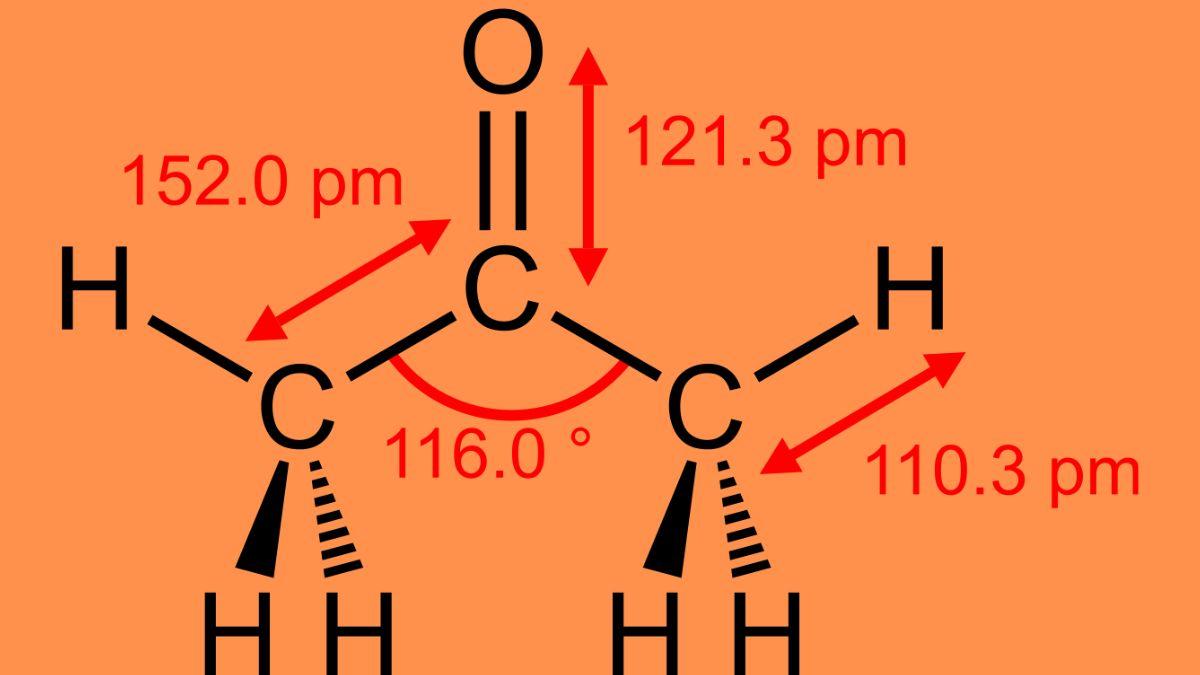
Structure of Propanone
The structural formula of propanone has been given below so that you can get an absolute clarity about the shape of the propanone. Additionally, the molecule’s center is planar-trigonal because of the C sp2. Contrarily, the methyl group at the extremes displays tetrahedral geometry. Additionally, organic molecules are commonly represented as follows:
Propanone Formula: General Properties
Check some of the general properties that the Propanone formula gives to propanone in the table below.
| Parameters | Details |
| Compound Name | Propanone |
| Common Name | Acetone |
| Propanone Formula (Chemical) | C3H6O |
| Appearance | Colourless liquid |
| Odor | Pungent, irritating, floral |
| Boiling Point | 56°C |
| Melting Point | -95°C |
| Molecular mass | 58.08 g/mol |
| Density | 0.7845 g/ml at 25 °C (room temperature) |
Preparation of Propanone
83% of propanone in the industry is manufactured using the cumene process. In the cumene process, benzene reacts with propylene to make cumene, which is then oxidized with air to yield phenol and acetone. The most popular way to produce propanone is through the oxidation of cumene. The process consists of two stages: Initially, benzene combines with propane to generate cumene, which is later oxidized with O2 to form cumene hydroperoxide (CHP). Following this, the catalyst caused a reaction that separated the CHP into phenol and propanone.

Physical Properties of Propanone
Propanone is a clear substance with a sugary smell and is a liquid that evaporates easily. Moreover, the freezing temperature is -94.9°C and the heating temperature is 56.08°C. The propanone has a density of 0.785 grams per milliliter. Additionally, it can dissolve in water, benzene, ether, dimethylformamide, and alcohol.
Chemical Properties of Propanone
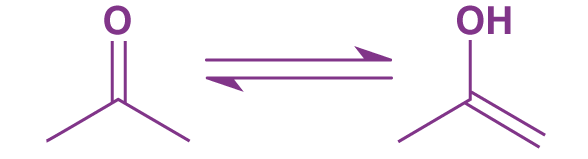
The propanone formula gives propanone special chemical properties. Having a carbonyl group –C=O at the center causes molecule polarization due to the lower electronegativity of carbon compared to oxygen. This is why we are able to utilize it as a reactant alongside nucleophilic molecules that target the carbonyl carbon lacking in electrons. The propanone also possess keto-enol tautomerism which is shown in the diagram below.
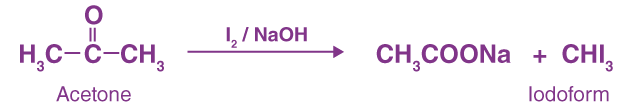
The haloform reaction of propanone occurs because of the CH3-C=O group; when propanone reacts with a halogen in the presence of an alkali, it produces a haloform and an acid salt.
Uses of Propanone
Some of the most common uses of propanone has been highlighted below.
- It is utilized as a solute for artificial fibers and plastics.
- It serves as a forerunner to methyl methacrylate.
- It is utilized for prepping metal prior to painting.
- It is employed in certain medications within the pharmaceutical sector.
- Because of its volatile nature, it is utilized in laboratories for cleaning lab glassware.
- It serves as a desiccant.
- It is employed during the de-fatting procedure.
- It is utilized in beauty products like nail polish remover.
- It is utilized for treating acne.
Health Hazards of Propanone
Just like any other chemical compound, the propane causes several side effects along with its usefulness. Propanone is very flammable, although it is generally considered to pose minimal risk for both immediate and long-term health effects. Inhaling acetone may result in a sore throat or cough. Check some of the health hazards of propanone below.
- Propanone is extremely flammable but is widely known to have low levels of both short-term and long-term toxicity. Breathing in acetone may lead to a throat irritation or cough.
- Inhaling moderate to high levels of propanone briefly can cause irritation in your nose, throat, lungs, and eyes.
- It can also lead to headaches, dizziness, confusion, increased heart rate, nausea, vomiting, blood abnormalities, loss of consciousness, potential coma, and a shortened menstrual cycle in females.
| Related Articles | |
| Carbonic Acid Formula | Titration Formula |
| Aluminium Fluoride Formula | Hydrofluoric acid formula |




 CUET Chemistry Syllabus 2025, Download O...
CUET Chemistry Syllabus 2025, Download O...
 ICSE Class 10 Chemistry 30 Day Preparati...
ICSE Class 10 Chemistry 30 Day Preparati...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...










