Table of Contents
Redox Reaction
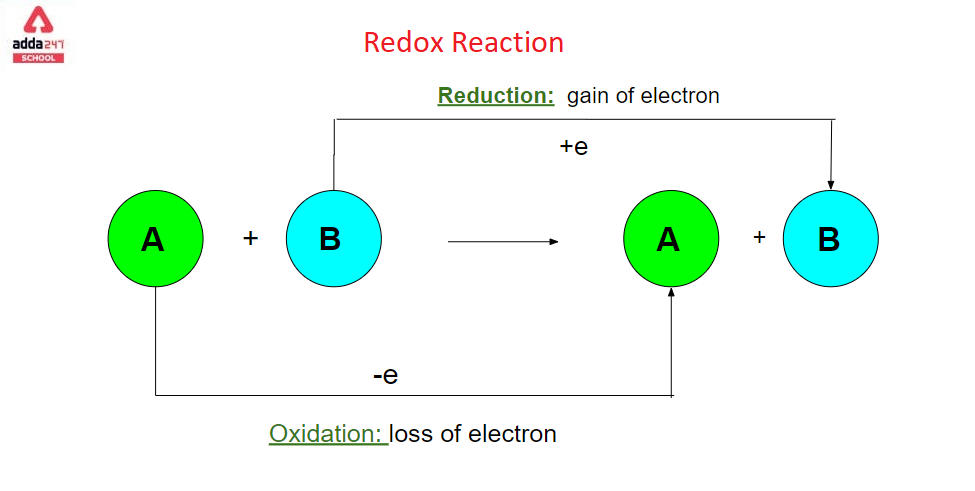
Redox reaction is a chemical reaction that involves changing the oxidation states of atoms. The actual or formal transfer of electrons between chemical species is characterised by redox reactions, which usually include one species (the reducing agent) suffering oxidation (losing electrons) while another species (the oxidising agent) undergoes reduction (gains electrons). [3] The chemical species that loses an electron is said to have been oxidised, while the chemical species that gains an electron is said to have been reduced. To put it another way:
Redox Reaction Definition
- The loss of electrons or an increase in the oxidation state of an atom, an ion, or specific atoms in a molecule is referred to as oxidation.
- A gain of electrons or a drop in the oxidation state of an atom, an ion, or specific atoms in a molecule is referred to as reduction (a reduction in oxidation state).
Redox Reactions- Types and Examples for Class 11
The following are types of redox reactions:
- Decomposition Reaction
- Combination Reaction
- Displacement Reaction
- Disproportionation Reactions
Decomposition Reaction
The breakdown of a molecule into other compounds is what this reaction entails. Various are some examples of these types of reactions:
2NaH → 2Na + H2
2H2O → 2H2 + O2
Na2CO3 → Na2O + CO2
All of the aforementioned reactions lead to the breakdown of smaller chemical compounds in the form of
AB → A + B
However, there is one exception that proves that not all decomposition reactions are redox reactions.
E. g.: CaCO3 → CaO + CO2
Combination Reaction
These reactions are the polar opposite of breakdown processes in that they combine two chemicals to generate a single compound in the form of a polymer A + B → AB.
E. g.: H2 + Cl2 → 2HCl
Displacement Reaction
An atom or an ion of a compound is replaced by an atom or an ion of another element in this reaction. It can be represented as X + YZ → XZ + Y. Displacement reactions can also be divided into two types.
E. g.: CuSO4+Zn→Cu+ZnSO4
- Metal displacement Reaction
- Non-metal displacement Reaction
Metal Displacement
A metal present in the compound is displaced by another metal in this reaction. In metallurgical procedures, when pure metals are extracted from their ores, several types of reactions are used.
Non-Metal Displacement
We can detect a hydrogen displacement reaction in this type of reaction, as well as rare oxygen displacement events.
Disproportionation Reactions
Disproportionation reactions are those that involve only one reactant being oxidized and reduced.
E.g.: P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3
How to do Redox Reaction Balancing?
By Oxidation Reaction
The addition of oxygen or the more electronegative element to a compound or the removal of hydrogen or the more electropositive element from a substance is called an oxidation reaction, according to one definition.
E. g.: 2S(s) + O2 (g) → SO2 (g) CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
By Reduction Reaction
Reduction reactions are defined as electron gains, just like oxidation reactions. Any material that gains an electron during a chemical reaction is decreased.
The addition of hydrogen or a more electropositive element to a substance, or the removal of a more electronegative element like oxygen, is known as a reduction reaction.
- g.: 2CH2CH2 (g) + H2 (g) → CH3CH3 (g)
Oxidizing and Reducing Agents
An oxidising agent is a material (atom, ion, or molecule) that acquires electrons and is thereby reduced to a low valency state. Eg: O2, O3, and X2 (halogens), etc. A reducing agent is a chemical that loses electrons and hence oxidises to a higher valency state. Eg: HI, H2S, Hydrogen, S, P, etc.
Related Post:
- NCC Full Form In Army, School, And College
- What Is The Valency Of Nitrogen?
- The Most Abundant Metal In The Earth’s Crust Is?
- Fleming’s Left Thumb Rule Statement
- Chlorine Atomic Mass And Weight In Kilo Grams
Redox Reaction: FAQs
Q. What are the 4 redox reactions?
Ans. Redox reactions are matched sets: if one species is oxidised, another must be reduced in the same process. As we look at the five primary types of redox reactions: combination, decomposition, displacement, combustion, and disproport, keep this in mind.
Q. Is respiration a redox reaction?
Ans. Cellular respiration is a redox reaction, which is an oxidation-reduction reaction. Respiration is a collection of metabolic reactions in which electrons are lost and gained. As a result, it’s referred to as the oxidation-reduction or redox reaction.
Q. Is glycolysis a redox reaction?
Ans. During glycolysis, there is only one redox reaction. During glycolysis, glucose is first oxidised. During the oxidation, NAD+ receives electrons and is reduced as a result. There are two NADHs generated in total.
Q. What is redox reaction in biology?
Ans. A redox reaction is a chemical reaction that involves both reduction and oxidation, resulting in changes in the oxidation numbers of the atoms involved. When the oxidation number increases, it is called oxidation; when the oxidation number decreases, it is called reduction.
Q. Is photosynthesis a redox reaction?
Ans. The conversion of light energy into chemical energy is known as photosynthesis. The primary event is a redox reaction involving light-driven electron transfer, which puts in motion a series of electron transfers on which all life eventually depends.
Q. Are redox reactions exergonic or endergonic?
Ans. The coupling of exergonic and endergonic events can be seen in oxidation-reduction (redox) reactions. Enzymes frequently work by linking an endergonic process with ATP exergonic hydrolysis.


 VITEEE Admit Card 2025 Out Today, Downlo...
VITEEE Admit Card 2025 Out Today, Downlo...
 NEET Organic Chemistry Syllabus 2025, Ch...
NEET Organic Chemistry Syllabus 2025, Ch...
 NTA NEET Exam Date 2025 OUT, Exam Timing...
NTA NEET Exam Date 2025 OUT, Exam Timing...










