Redox Reaction Notes: The Redox Reaction notes are of utmost importance for students as the concept is integral to understanding different chemical processes in the world of chemistry. The acceptance and loss of electrons is a highly important concept that explains different phenomena in the chemical world, including the acid-base properties of elements. This concept indirectly helps to predict and understand different chemical processes of both inorganic as well as organic chemistry. Most students are required to understand this concept beforehand starting their preparation for a competitive exam like NEET. To aid students in their preparation journey, we have created notes for this chapter in PDF form.
Redox Reaction Notes
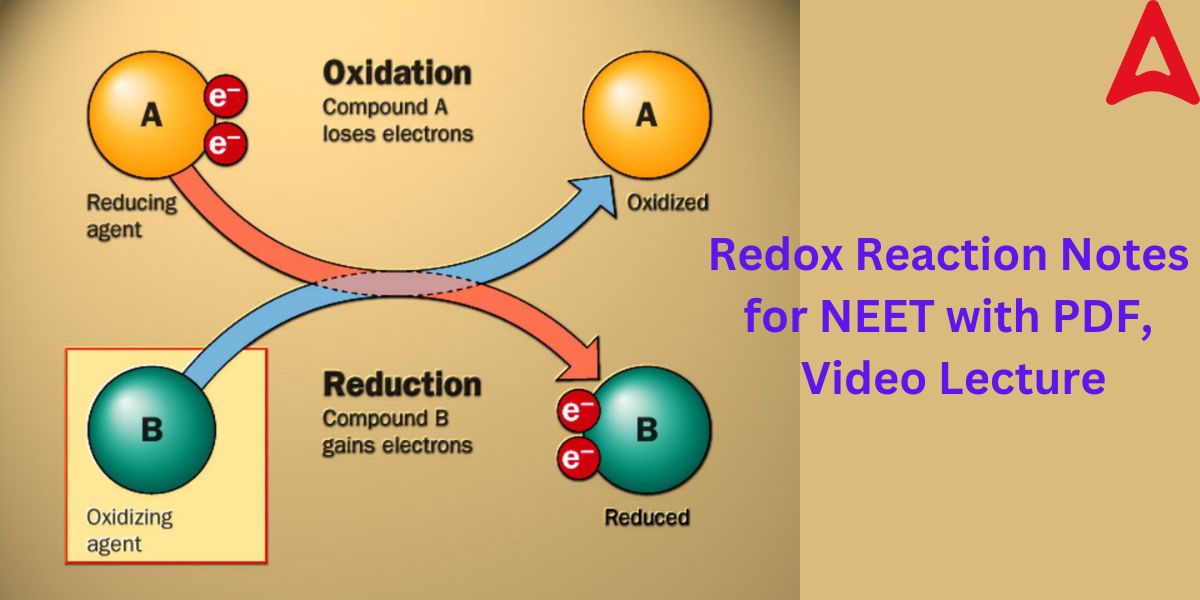
To gain a complete understanding of the Redox reaction, one must go through its detailed notes. Essentially, redox reactions are chemical processes involving oxidation and reduction in which the oxidation states of the reactants change. Redox is a shortened version of reduction-oxidation. A reduction process and an oxidation process are the two distinct processes that comprise all redox reactions. In redox or oxidation-reduction processes, the oxidation and reduction reactions usually take place concurrently. In a chemical reaction, the material that is being reduced is referred to as the oxidizing agent, and the substance that is being oxidized is referred to as the reducing agent.
Redox Reaction Class 11 Notes
Redox reaction is an important chapter in the Chemistry NCERT textbook of class 11th. The topic has been introduced in the 11th class itself so that students can understand all other related concepts easily thereafter in their preparation journey of the competitive exam. This topic is included in the 8th chapter of the NCERT Chemistry text book for 11th class. Understanding the traditional concept of Redox Reactions—which encompasses reduction and oxidation reactions as well as other topics like electrode processes, oxidation number, and electron transfer reactions is the main goal of this chapter. Some important notes on the redox reaction for class 11 is given below.
Redox Reaction Meaning
Any chemical reaction in which electrons are exchanged between the two reactants involved is referred to as a redox reaction. By monitoring the alterations in the oxidation states of the interacting species, this electron transfer can be detected. Oxidation is the process of a reactant losing electrons and increasing in oxidation state as a result. Reduction is the process of an electron being gained and the oxidation state of a reactant being decreased as a result.
Redox Reaction Types
The Redox Reaction can be categorized into 4 types.
- Combination Reaction
- Decomposition Reaction
- Disproportionation Reaction
- Displacement Reaction
1) Combination Reaction
These reactions require the combining of two compounds to generate a single compound in the form of A + B → AB. They are the opposite of decomposition reactions. Some of the examples of combination reactions are:
C+ O2→ CO2
H2 + Cl2 → 2HCl
4Fe + 3O2→ 2Fe2O3
2) Decomposition Reaction
In this type of reaction, a molecule breaks down into other compounds. These are opposite to that of combination reactions. Some examples of these kinds of reactions are
2H2O → 2H2 + O2
2NaH → 2Na + H2
Na2CO3 → Na2O + CO2
It is important to note that not all redox reactions correspond to redox reactions. In some special cases, the compound decomposes without resulting into any oxidation or reduction. The example of this special case is:
CaCO3 → CaO + CO2
3) Disproportionation Reaction
Those reactions in which a single reactant is oxidized and reduced is known as disproportionation reaction. The example of this type of redox reaction is given herein.
P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3
4) Displacement Reaction
An atom or an ion from one element is substituted for an atom or an ion from another in this type of reaction. It can be shown as follows: X + YZ → XZ + Y. These are further of two types – Metal Displacement reaction and Non-metal Displacement reaction. One metal in the combination gets replaced by another metal in the metal Displacement reaction. These kinds of reactions are used in metallurgical procedures to extract pure metals from their ores. In the Non-metal displacement kind of reaction, mostly the displacement of hydrogen takes place. On occasion, very infrequent reactions involving the displacement of oxygen can also take place. The example of the metal displacement reaction is given below.
CuSO4+Zn→Cu+ZnSO4
Oxidizing Agents
An oxidizing agent is any material (atom, ion, or molecule) that absorbs electrons and is therefore reduced to a low valency state. Oxidizing agents are generally electronegative elements, oxides of metals, and oxide of non-metals. In this world, the strongest oxidizing agent is Fluorine. Some examples of oxidizing agents are O2, O3, KMnO4, HNO3, CuO, MgO, etc.
Reducing Agents
A reducing agent is a material that loses electrons and is oxidized to a higher valency state as a result. Reducing agents are generally metals, hydracids, metallic compounds, etc. Some examples of Reducing agents are Zn, Na, HI, HCl, LiH, NaH, etc.
Redox Reaction During Combustion
As a kind of oxidation-reduction reaction, combustion is classified as a redox reaction. Since an explosion is a quick type of combustion, it can be thought of as a redox reaction. Redox reactions are used even aboard the space shuttle. An oxidation-reduction reaction results from the mixture of ammonium perchlorate and powdered aluminum inside the rocket boosters.
Redox Reaction Application
Some real life applications of the redox reaction are given below.
- Redox reactions are used in the electroplating process to apply a thin layer of material to an item. The process of electroplating is used to create jewelry with a gold finish.
- Redox processes are used to extract a lot of metals from their ores. Smelting metal sulfides in the presence of reducing chemicals is one instance of this.
- Electrolysis, which is dependent on redox processes, is also a key component in the production of several significant compounds. Redox reactions are used in the production of many chemicals, such as caustic soda, chlorine, etc.
- Reactions of oxidation and reduction are also used to bleach things and sanitize water.
- Many metals can have their surfaces shielded from corrosion by attaching them to sacrificial anodes, which experience corrosion in their place. A typical use of this process is the galvanization of steel.
- Oxidation is a process used in the industrial manufacturing of cleaning products.
- A common ingredient in fertilizers, nitric acid is created when ammonia undergoes an oxidation process.
Redox Reaction Notes for NEET
Redox reaction is an important chapter for the NEET exam. Every year, around 1-2 questions (2% to 4% of the sectional questions) come from this topic directly. The concept of this topic indirectly appears in many other questions of chemistry. So , we can understand the high importance of this chapter. To help students with their preparation, we have created the notes PDF for this chapter especially for NEET aspirants. Students can download the PDF by clicking the link given below.
Redox Reaction Notes for NEET PDF Part 1
Redox Reaction Notes for NEET PDF Part 2
Redox Reaction Video Lecture
The video lecture of all the detailed concept on this chapter is given hereunder. Students should go through this video to get a full clarity of Redox reaction as per the NEET exam standards.










 Chemistry Investigatory Project Class 12...
Chemistry Investigatory Project Class 12...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...
 CUET Chemistry Syllabus 2026, Download O...
CUET Chemistry Syllabus 2026, Download O...














