Table of Contents
Reimer Tiemann Reaction: The ortho-formylation of C6H5OH (phenols) is accomplished via the Reimer Tiemann reaction. Under normal circumstances, the Reimer-Tiemann reaction occurs, which is an aromatic substitution reaction. The reaction was named after Karl Reimer and Ferdinand Tiemann, two scientists. Reimer Tiemann reaction is one of the few organic reactions whose industrial importance appears to grow year after year, despite the environmental risks connected with the usage of chloroform. Dichlorocarbene is a Reimer Tiemann reaction intermediate formed by the reaction of chloroform with base.
Reimer Tiemann Reaction
What is Reimer Tiemann Reaction?
In the Reimer Tiemann reaction, ortho- or para- formylated products are produced by heating phenol or other electron-rich aromatic compounds like naphthols, pyrroles, indoles, etc. with chloroform and an aqueous alkali.
While phenol, i.e. C6H5OH, undergoes a reaction with CHCl3 (chloroform) in the presence of NaOH (sodium hydroxide), an aldehyde group (-CHO) is inserted at the ortho position of the benzene ring, resulting in the creation of o-hydroxybenzaldehyde. The reaction is commonly referred to as the Reimer Tiemann reaction.
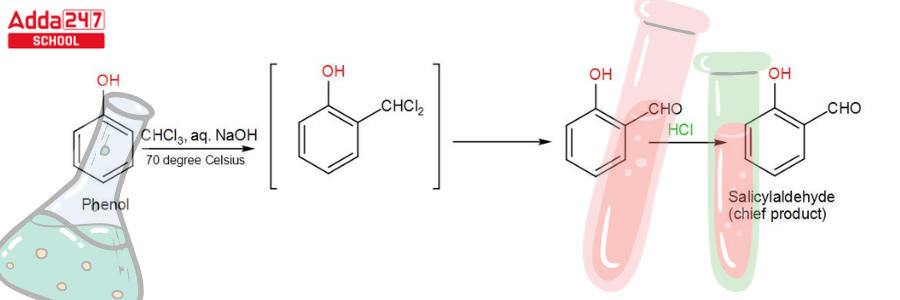
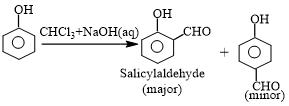
This reaction produces a mixture of ortho and para isomers, with the ortho isomer being the predominant result. The para-isomer is the main product if any of the ortho positions of the benzene ring is occupied. The two isomers are separated via fractional distillation, which involves distilling unreacted phenol and the ortho-isomer over the para-isomer.
Reimer Tiemann Reaction Mechanism
The Reimer Tiemann reaction requires heat to begin, and once it begins, it can result in a highly exothermic reaction. This boosts the reaction rate even further. This is why, in thermal runaways, Reimer Tiemann reactions are always preferable. The Reimer Tiemann reaction mechanism is explored further below.
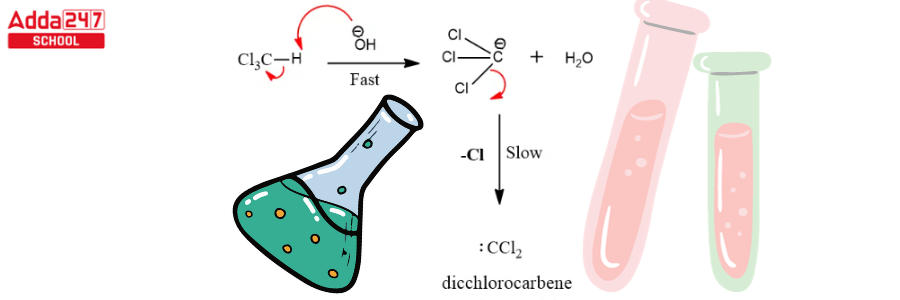
- The chloroform carbanion is formed when the chloroform is deprotonated by the strongly basic aqueous hydroxide solution.
- This chloroform carbanion is simply eliminated by alpha elimination, yielding dichlorocarbene as the result. The major reactive species, as previously stated, is dichlorocarbene.
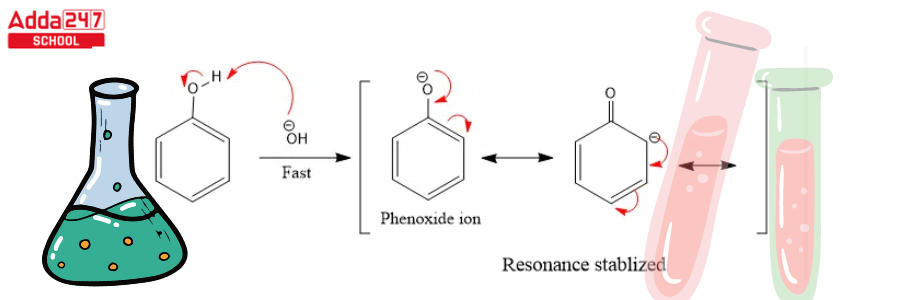
- The phenol reactant is then deprotonated by the aqueous hydroxide, yielding a negatively charged phenoxide.
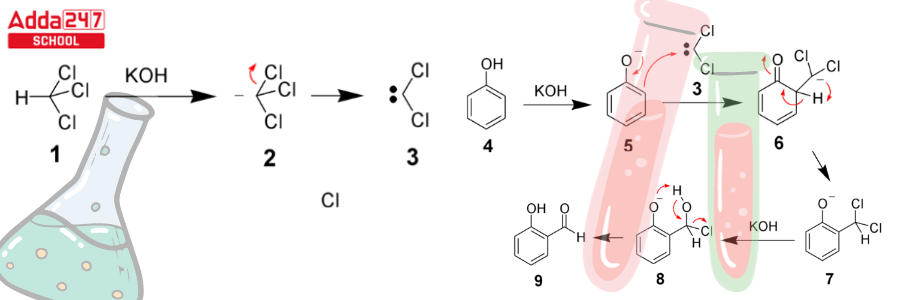
- This negative charge has now been delocalized within the benzene ring, making it significantly more nucleophilic.
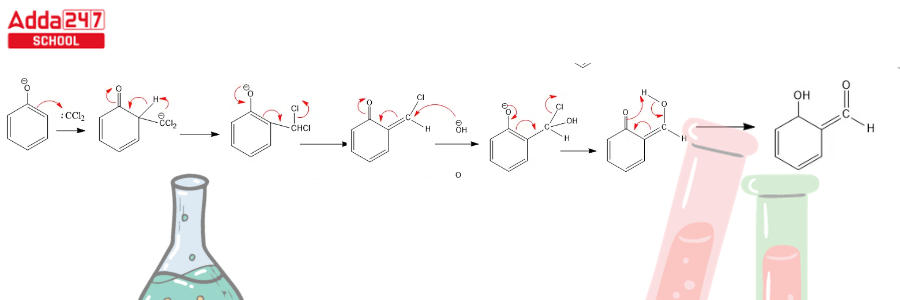
- As a result, the dichlorocarbene undergoes a nucleophilic attack, resulting in the formation of an intermediate dichloromethyl-substituted phenol.
- This intermediate is subjected to basic hydrolysis in order to provide the required ortho-hydroxybenzaldehyde.
Reimer Tiemann Reaction of Phenol
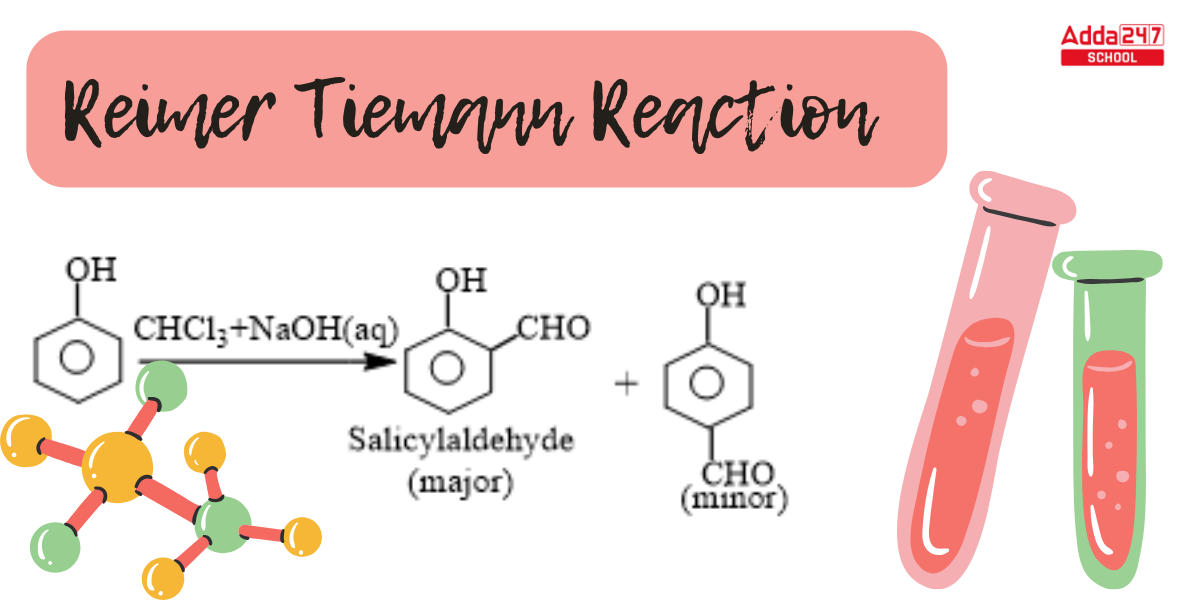
When phenol combines with chloroform in an atmosphere of bases (NaOH/KOH), an aldehyde (CHO) group is inserted in the ring ortho to the phenolic group, resulting in salicylaldehyde. This is known as the Reimer-Tiemann reaction.
Dichlorocarbene serves as a reactive intermediate in the Reimer Tiemann reaction. The next stages complete this reaction.
Step 1: Create a carbene that works as an electrophile.
Step 2: The base extracts the hydrogen atom from the -OH group to generate the resonance stabilised phenoxide ion.
Step 3: Dichlorocarbene addition to the ring, followed by hydrolysis
Things to Remember about Reimer Tiemann Reaction
- It is an example of a substitution reaction.
- This process is mostly used to produce salicylaldehyde from phenol.
- It must be done in a biphasic solvent system. A biphasic mixture is an amalgamation of immiscible stages, most typically a natural solvent and an aqueous phase.
- The reaction is very exothermic once it has commenced.
- Rapid-fire mixing, phase transfer catalysts, or the use of 1,4-Dioxane, an emulsifying agent, are used to combine the two chemicals.
- When additional hydroxy-aromatic chemicals, such as naphthols, are employed, this reaction is effective.
- Heat is required to initiate the chemical process.
- Salicylaldehyde is the result of the Reimer Tiemann reactions when phenol, chloroform, and alkali are employed. Salicylic acid is produced when phenol, CCl4, and alkali are combined.
Reimer Tiemann Reaction Application
The Reimer Tiemann reaction is used in the following ways:
- Primarily Reimer Tiemann reaction is used in the ortho formulation of phenols.
- It can be used to make 3-halo pyridines, naphthalenes, quinolines, and other compounds.
- Reimer-Tiemann reactions are frequently slightly modified to produce phenolic acids such as 2-hydroxybenzoic acid by replacing the chloroform with carbon tetrachloride.
- Reimer Tiemann reaction can be used to make Salicyaldehyde (Salicyladehyde is made by reacting phenol with chloroform in the presence of an alkali such as NaOH or KOH), which is used in perfumes, as a flavoring ingredient, and for medical purposes.
- The direct formylation of aromatic compounds via the Reimer-Tiemann reaction is typically done safely and is the simplest approach for formylation because it is the only method that does not require acidic or anhydrous conditions.
Limitations of Reimer Tiemann Reaction
The following are the limitations of the Reimer Tiemann Reaction.
- Aromatic aldehyde yields are low.
- Because of the two-phase reaction system, mass transfer between two layers is insufficient.
Reimer Tiemann Reaction with Example
The Reimer-Tiemann reaction is a chemical reaction that converts phenols to salicylaldehydes (ortho-hydroxybenzaldehydes) by treating them with chloroform (CHCl3) and a strong base, typically sodium hydroxide (NaOH) or potassium hydroxide (KOH). Here is the general reaction:
Reimer-Tiemann Reaction:
Phenol + Chloroform + Strong Base → Salicylaldehyde
Here’s an example of the Reimer-Tiemann reaction using phenol:
Reaction:
Phenol (C6H5OH) + Chloroform (CHCl3) + Sodium Hydroxide (NaOH) → Salicylaldehyde (2-Hydroxybenzaldehyde)
Mechanism:
The reaction mechanism involves the following steps:
- The strong base (NaOH) deprotonates the phenol, forming the phenoxide ion.C6H5OH + NaOH → C6H5O-Na+ + H2O
- The phenoxide ion reacts with chloroform, which is activated by the base, leading to the formation of an intermediate called the dichlorocarbene.C6H5O-Na+ + CHCl3 → C6H4OCCl2-Na+ + HCl
- The dichlorocarbene rearranges to form salicylaldehyde through a series of intramolecular reactions.C6H4OCCl2-Na+ → Salicylaldehyde + NaCl
Salicylaldehyde is an important compound in organic synthesis and is used as an intermediate in the production of various pharmaceuticals and other organic compounds.
Reimer Tiemann Reaction in Hindi
रेइमर-टीमैन प्रतिक्रिया एक रासायनिक प्रतिक्रिया है जो क्लोरोफॉर्म (सीएचसीएल 3) और एक मजबूत आधार, आमतौर पर सोडियम हाइड्रॉक्साइड (NaOH) या पोटेशियम हाइड्रॉक्साइड (KOH) के साथ उपचार करके फिनोल को सैलिसिल्डिहाइड (ऑर्थो-हाइड्रॉक्सीबेंज़ाल्डिहाइड) में परिवर्तित करती है। यहाँ सामान्य प्रतिक्रिया है:
रीमर-टीमैन प्रतिक्रिया:
फिनोल + क्लोरोफॉर्म + मजबूत आधार → सैलिसिलैल्डिहाइड
यहां फिनोल का उपयोग करके रेइमर-टीमैन प्रतिक्रिया का एक उदाहरण दिया गया है:
प्रतिक्रिया:
फिनोल (C6H5OH) + क्लोरोफॉर्म (CHCl3) + सोडियम हाइड्रॉक्साइड (NaOH) → सैलिसिलैल्डिहाइड (2-हाइड्रोक्सीबेंजाल्डिहाइड)
तंत्र:
प्रतिक्रिया तंत्र में निम्नलिखित चरण शामिल हैं:
मजबूत आधार (NaOH) फिनोल को अवक्षेपित करता है, जिससे फेनॉक्साइड आयन बनता है।
C6H5OH + NaOH → C6H5O-Na+ + H2O
फेनोक्साइड आयन क्लोरोफॉर्म के साथ प्रतिक्रिया करता है, जो आधार द्वारा सक्रिय होता है, जिससे डाइक्लोरोकार्बिन नामक एक मध्यवर्ती का निर्माण होता है।
C6H5O-Na+ + CHCl3 → C6H4OCCl2-Na+ + HCl
डाइक्लोरोकार्बिन इंट्रामोल्युलर प्रतिक्रियाओं की एक श्रृंखला के माध्यम से सैलिसिलैल्डिहाइड बनाने के लिए पुनर्व्यवस्थित होता है।
C6H4OCCl2-Na+ → सैलिसिलैल्डिहाइड + NaCl
सैलिसिलैल्डिहाइड कार्बनिक संश्लेषण में एक महत्वपूर्ण यौगिक है और इसका उपयोग विभिन्न फार्मास्यूटिकल्स और अन्य कार्बनिक यौगिकों के उत्पादन में एक मध्यवर्ती के रूप में किया जाता है।


 NEET City Intimation Slip 2025 Available...
NEET City Intimation Slip 2025 Available...
 Assam HS Result 2025 Out Today, Check AH...
Assam HS Result 2025 Out Today, Check AH...
 TS 10th Results 2025 OUT Today, Check BS...
TS 10th Results 2025 OUT Today, Check BS...










