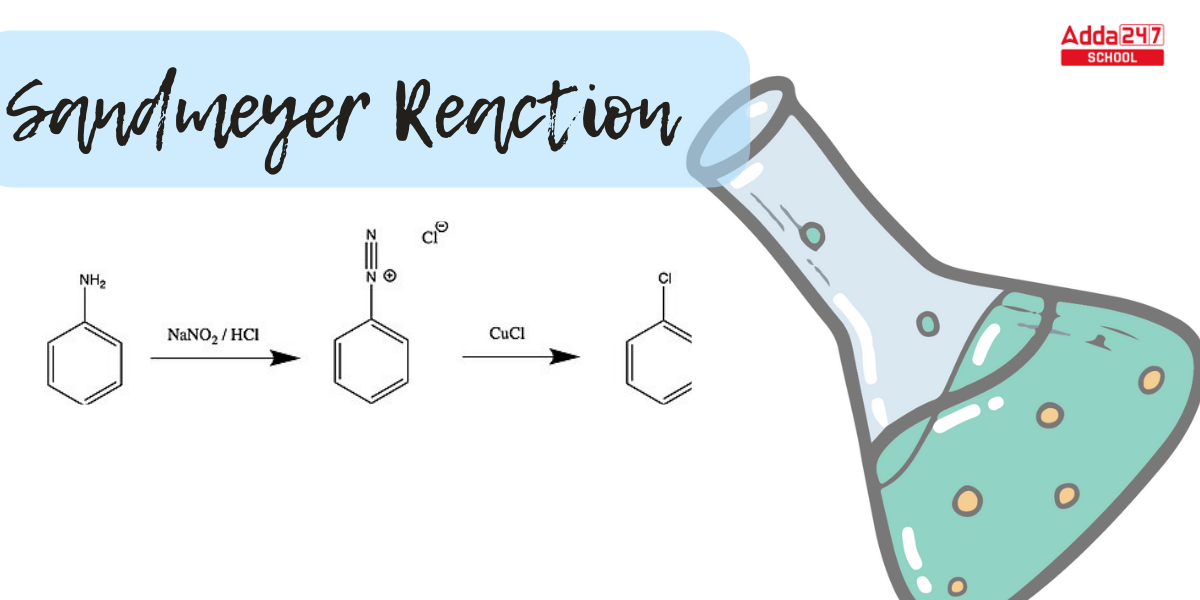
Sandmeyer Reaction: In aromatic chemistry, the Sandmeyer Reaction is a crucial transition. The Sandmeyer reaction is an aromatic radical-nucleophilic substitution process. It is critical because it can result in substitution sequences that are not possible with direct substitution. Sandmeyer Reaction is a convenient tool for replacing an amino group on an aromatic ring with different substituents. Let us study more about the Sandmeyer Reaction mechanism, examples of reactions, and the significance of the Sandmeyer Reaction in organic chemistry.
Sandmeyer Reaction
What is Sandmeyer’s Reaction? The Sandmeyer reaction produces aryl halides from aryl diazonium salts. Traugott Sandmeyer, a Swiss scientist, discovered the reaction in 1884 while attempting to develop phenylacetylene from benzene diazonium chloride and cuprous acetylide; at the end of the experiment, he received phenyl chloride as the major result.
The amino group is turned into a diazonium salt during the Sandmeyer reaction, which can then be changed into other functional groups employing a catalyst. In this reaction, copper salts such as chloride, bromide, or iodide ions act as catalysts. Notably, the Sandmeyer reaction can be exploited to perform novel benzene transformations.
Mechanism of Sandmeyer Reaction
Sandmeyer reaction helps in the synthesis of aryl halides from aryl diazonium salts. This is a radical-nucleophilic aromatic substitution. Several processes are involved in the Sandmeyer reaction mechanism, including the formation of intermediate molecules are discussed below.
Step 1: Protonation occurs twice, with sodium nitrite reacting with hydrogen halide to produce nitrosonium and halide ions.
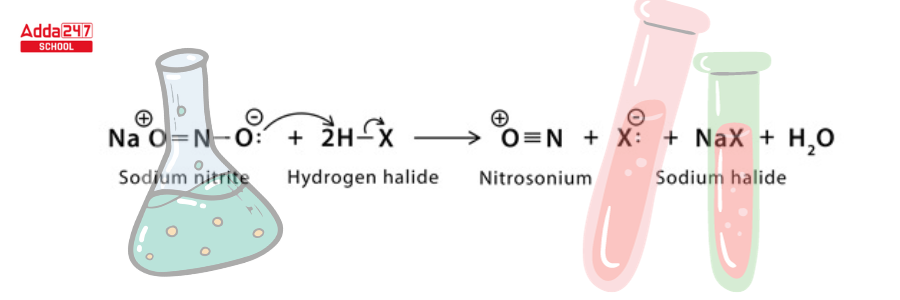
Step 2: The electrophilic nitrosonium converts amine to diazonium salt.
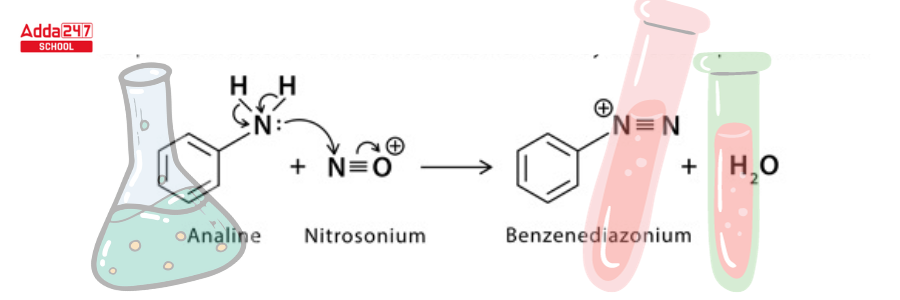
Step 3: A single electron transfer from copper to diazonium and from halide to copper (1) halide ion results in the formation of a neutral diazo radical and copper (II) halide.
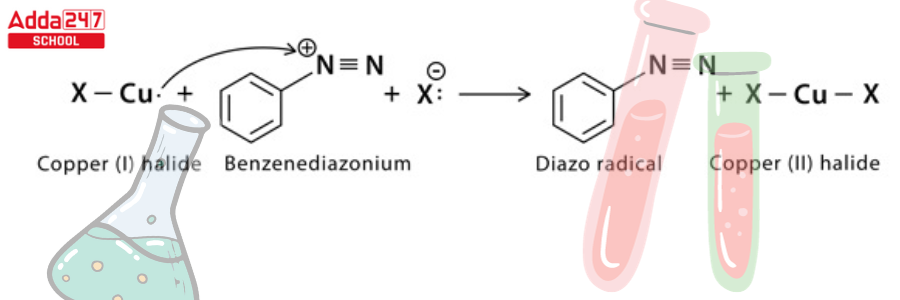
Step 4: The diazo radical releases nitrogen gas to form an aryl radical, which combines with the copper (II) halide to regenerate the copper (1) halide catalyst and form the final aryl halide product.
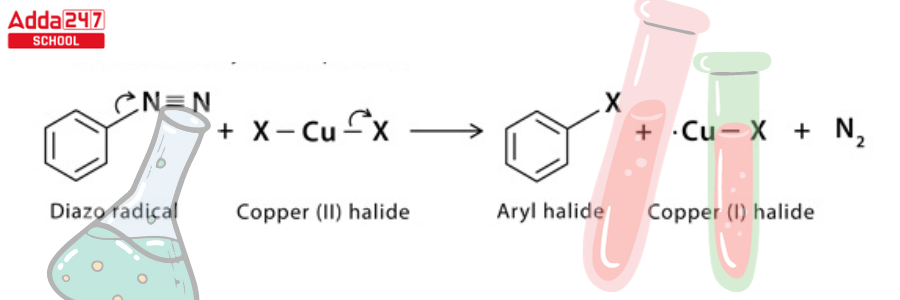
Sandmeyer Reaction Example
Sandmeyer reaction is a radical-nucleophilic aromatic replacement. The Sandmeyer reaction uses a two-step mechanism. The Sandmeyer reaction is a free radical reaction.
- The halogen linked to copper joins the benzene ring during this process.
- When diazonium salts are combined with CuCl dissolved in HCl or CuBr dissolved in HBr, the Sandmeyer reaction occurs, resulting in the synthesis of aryl chloride or aryl bromide.
- The Sandmeyer reaction is expressed as follows:
- stage 1 – sodium nitrite is converted into nitrous acid.
NaNO2+HCl(dil)→HONO+NaCl
-
- Step 2 – Nitrous acid then interacts with aniline to produce diazonium salt.
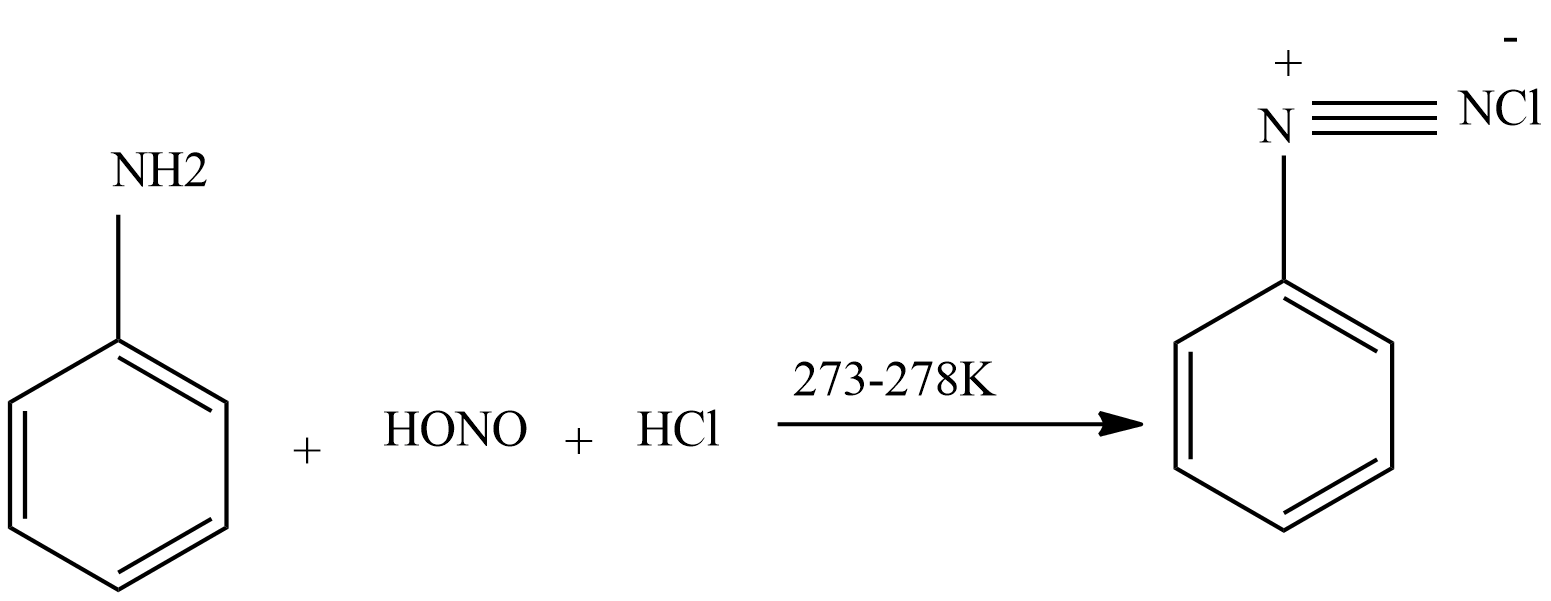
-
- Step 3 – Diazonium salt then interacts with CuCl dissolved in HCl to produce chloroarenes.
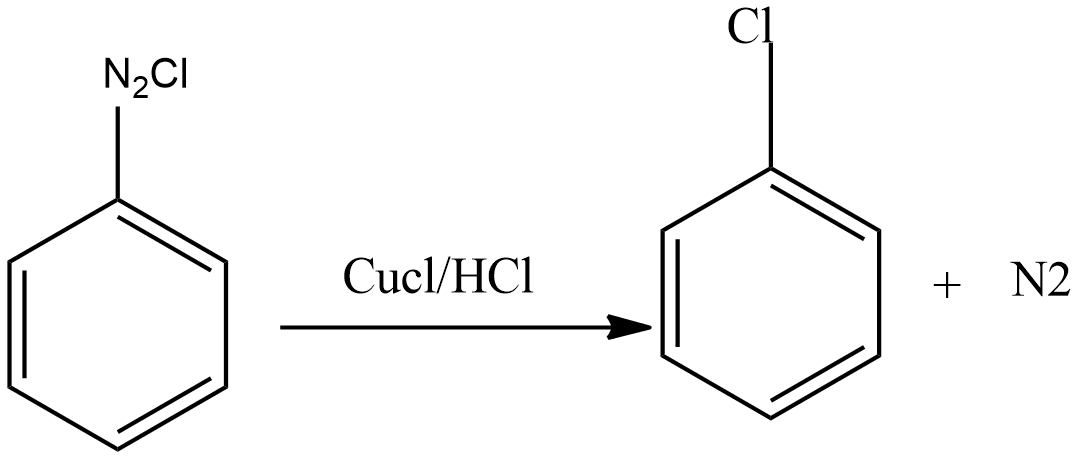
Sandmeyer Reaction and Gattermann reaction
Sandmeyer reaction –
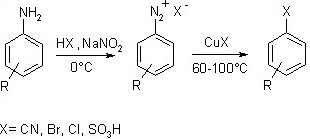
This reaction produces aryl halides from aryl diazonium ions. The Sandmeyer reaction employs copper as a catalyst. All of these reactions are radical-nucleophilic aromatic substitution reactions, which include benzene hydroxylation, trifluoromethylation, cyanation, halogenations, and so on.
In this reaction, aniline is treated with a mixture of NaNO2 and HCl to produce benzene diazonium chloride, which is then reacted with cuprous salts to produce haloarenes.
Gattermann Reaction –
In this procedure, aniline is first transformed into diazonium salt by treating it with a NaNO2 and HCl combination. The diazonium salt is then heated with HCl or HBr in the presence of copper metal to produce haloarenes.
Difference Between Sandmeyer reaction and Gattermann Reaction –
The sole difference between Sandmeyer and Gattermann reactions is their reactants. The reactants in Sandmeyer’s reaction are cuprous halide and halogen acid, which are Cucl/HCl or CuBr/HBr, whereas the reactants in Gattermann’s reaction are mixes of copper salt and halogen acid, which are Cu/HCl or Cu/HBr.
Similarities –
The process of transformation of aniline into diazonium salt is a feature shared by Sandmeyer’s and Gattermann’s reactions.
Significance of Sandmeyer Reaction
When researching aromatic chemistry, the Sandmeyer reaction is crucial. The significant of the Sandmeyer reaction is discussed below.
- Synthesis of aryl halide Di-iodomethane is utilized in the synthesis of aryl iodides. Bromoform is a chemical that is utilized in the production of aryl bromides. Aryl chlorides are created using chloroform.
- It uses an electron-transfer mechanism that includes free radicals.
- This process is employed in hydroxylation to convert aryl amines to phenols, resulting in the creation of an aryl diazonium salt.
- The Sandmeyer reaction has broad synthetic applications and is useful in conjunction with electrophilic aromatic substitution.
Sandmeyer Reaction Applications
The Sandmeyer reaction has a wide range of synthetic uses. There have been numerous versions of the Sandmeyer reaction produced.
Halogenation – Halogenation is the process by which aryl halides are synthesized. Some of the solvents utilized in this method are diiodomethane for aryl iodides, bromoform for aryl bromides, and chloroform for aryl chlorides.
Cyanation – The Sandmeyer reaction is employed in the cyanation process to produce benzonitriles. The reaction is also discovered during the manufacture of neoamphimedine, a chemical molecule used as an anti-cancer medication that targets topoisomerase II. Synthesis of benzonitriles and other important pharmaceuticals
Trifluoromethylation – Trifluoromethylation is the process by which aryl compounds with a trifluoromethyl group are synthesized and used as medicines.
Hydroxylation – It is employed in hydroxylation to convert aryl amines to phenols, which results in the creation of an aryl diazonium salt.








 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...
 CUET PG Crash Course 2026: Subject-Wise ...
CUET PG Crash Course 2026: Subject-Wise ...














