Table of Contents
Thermodynamics is an important concept that is common in the realm of physics and chemistry. It plays an important role in understanding various interrelated concepts of chemistry and physics. Understanding the movement of heat and energy in a system is a crucial topic for higher education. The kinetics of internal energy involving heat and the changes from one form of energy to the other are the concepts studied in the thermodynamics chapter. This chapter holds a significant weightage in competitive exams like NEET. To help students learn this chapter thoroughly, we have explained the second law of thermodynamics in this article. For better and more detailed learning, we have also included the thermodynamics notes PDF and its lecture as per the NEET chemistry syllabus.
Second Law of Thermodynamics
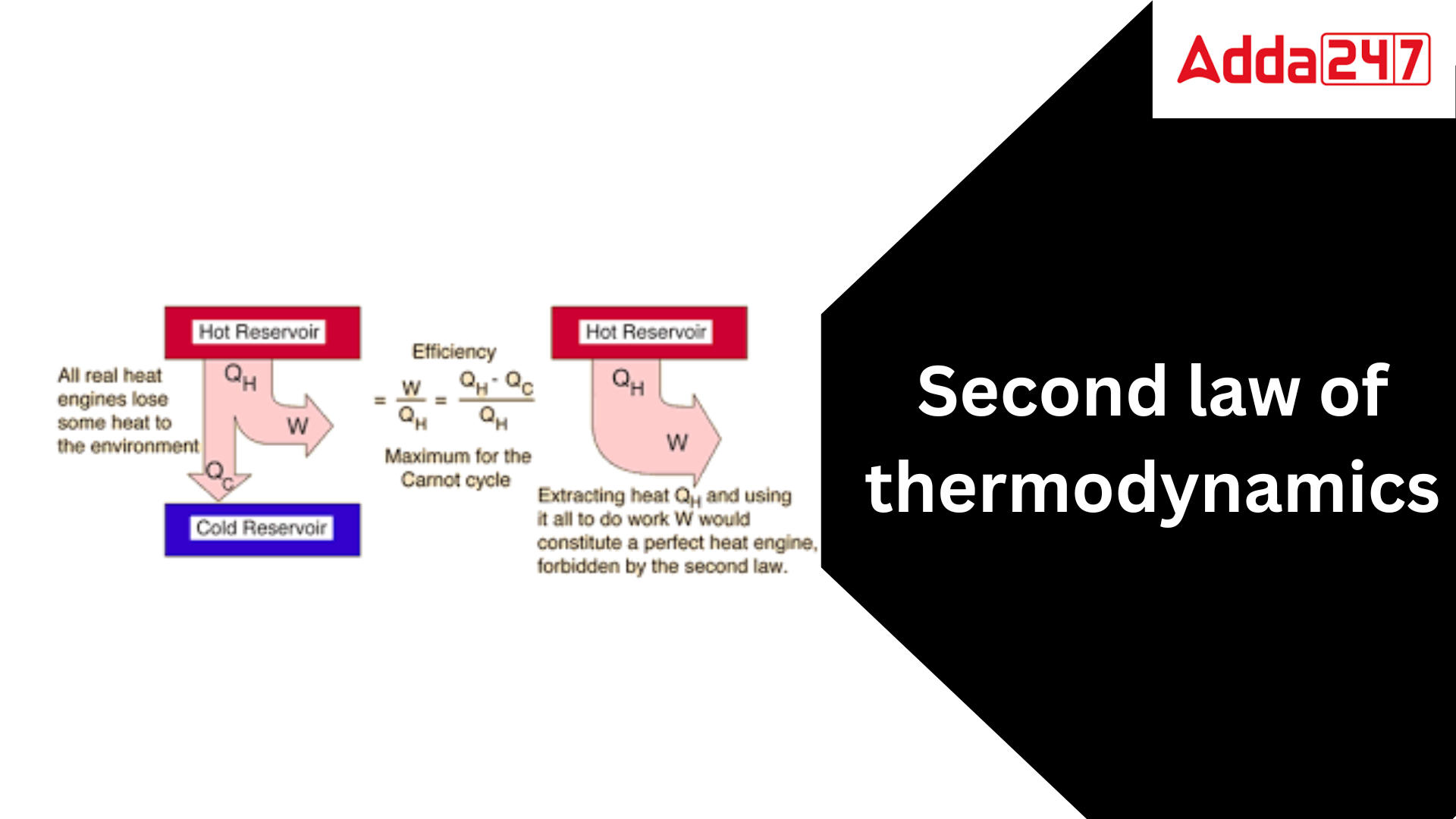
The second rule of thermodynamics limits the direction of heat transmission and the achievable efficiency of heat engines. The first law of thermodynamics asserts that the energy of the universe remains constant; while energy can be exchanged between system and surroundings, it cannot be created or destroyed. While the first law of thermodynamics provides information on the quantity of energy transferred as a process, it does not provide any information about the direction of energy transfer or the quality of the energy. The first law does not specify whether a metallic bar of uniform temperature can spontaneously become warmer at one end and cooler at the other. All the law can say is that if the process occurs, there will always be an energy balance. The second law of thermodynamics offers the criterion for the viability of any process. A process cannot occur unless it obeys both the first and second laws of thermodynamics.
Second Law of Thermodynamics Definition
The Second statement of Thermodynamics is a physical statement that states that the entropy of an isolated system always increases over time. Entropy is a measure of disorder, and the Second Law of Thermodynamics states that the cosmos becomes more disordered with time. This suggests that it is impossible to build a flawless perpetual motion machine and that all processes in the universe are irreversible. The Second Law of Thermodynamics has numerous ramifications for our knowledge of the universe. It explains, for example, why heat always travels from hot to cold and why it is difficult to totally transform heat into work. It also has consequences for the cosmos’s future, as it indicates that the universe will eventually reach a state of maximum entropy, at which point all processes would have ended. The Second Law of Thermodynamics is one of the most important principles in physics, and it has had a significant impact on our understanding of the universe. It is a law that is still being studied and disputed today, and it is likely to remain a vital element of our knowledge of the universe for many years to come.
Second Law of Thermodynamics Formula
The second law of thermodynamics is denoted mathematically as
ΔSuniv > 0
Where ΔSuniv is the change in the entropy of the cosmos.
Entropy is a measure of the randomness of a system, or it is a measure of energy or chaos within an isolated system. It can be thought of as a quantitative index that describes the quality of energy.
Meanwhile, a few things enhance the entropy of the closed system. To begin, in a closed system, while the mass remains constant, there is an exchange of heat with the surroundings. This change in heat content causes a disruption in the system, increasing its entropy.
Second, intrinsic alterations in the movement of the system’s molecules may occur. This causes perturbations, which induce irreversibilities inside the system, increasing its entropy.
Different Statements of the Law for second law of thermodynamics
Here are some distinct statements of the Second Law of Thermodynamics:
- Clausius’ statement: It is difficult to transmit heat from a colder to a hotter body without doing labour.
- According to the Kelvin-Planck equation, it is impossible to build a heat engine that, when working in a cycle, takes in heat from a single reservoir and produces an equivalent amount of work without rejecting any heat to a cooler reservoir.Exceptions:If Q2 = 0 (i.e., Wnet = Q1, or efficiency = 1.00), the heat engine performs work in a complete cycle by exchanging heat with only one reservoir, breaking the Kelvin-Planck equation.
- Carnot statement: No heat engine operating between two given temperatures can be more efficient than a Carnot heat engine working between the same two temperatures.
These statements are all comparable and mean the same thing. They all claim that it is impossible to build a perfect perpetual motion machine and that all processes in the cosmos are irreversible.
The Second Law of Thermodynamics is one of the most important principles in physics, and it has had a significant impact on our understanding of the universe. It is a law that is still being studied and disputed today, and it is likely to remain a vital element of our knowledge of the universe for many years to come.
The table below consists of Different Statements of the Law for second law of thermodynamics.
| Statement | Author | Year |
|---|---|---|
| Clausius statement | Rudolf Clausius | 1850 |
| Kelvin-Planck statement | Lord Kelvin and Max Planck | 1851 and 1901 |
| Carnot statement | Nicolas Léonard Sadi Carnot | 1824 |
Second law of Thermodynamics in Hindi
द्वितीय तापमानकीय कानून (Second Law of Thermodynamics) एक महत्वपूर्ण भौतिकीय कानून है जो उष्मीय सम्प्रवाह और ऊष्मीय संचय के संबंध में बताता है। यह कानून कहता है कि प्राकृतिक प्रक्रियाएं ऐसी होती हैं जिनके दौरान ऊष्मा एक से अधिक स्थानों में से गर्म स्थान से शीत स्थान की ओर प्रवेश करती है। इसका मतलब है कि ऊष्मा का प्रवाह सदैव गर्मी स्थान से शीत स्थान की ओर होता है और कभी भी विपरीत नहीं होता है।
इस कानून का महत्वपूर्ण प्रयोजन बताना है कि प्रकृति में ऊष्मीय संचय के लिए युक्तियुक्त प्रक्रियाएं होती हैं, परन्तु इसे व्यक्तिगत अभिव्यक्ति में नहीं देखा जा सकता है। यह भौतिकी के लिए एक महत्वपूर्ण नियम है जो अध्ययन, विज्ञान, और इंजीनियरिंग में उपयोगी है।
द्वितीय तापमानकीय कानून के अनुसार, यह प्रक्रियाएं समय के साथ बदलती रहती हैं और इन प्रक्रियाओं का व्यवहार एक प्रकार से विशिष्ट दिशा में होता है, जिससे ऊष्मा की अवगति होती है। उदाहरण के लिए, एक गर्म बरतन से उठने वाली गर्म वायु का प्रवाह ठंडे इलाके की ओर होगा। इसी प्रकार, एक आधुनिक इंजन में ऊष्मा को बदलकर उसका उपयोग मैकेनिकल शक्ति में परिवर्तित किया जा सकता है।
इस नियम का प्रमुख उपयोग विभिन्न तकनीकी प्रक्रियाओं, ऊर्जा उत्पादन और ऊर्जा बचत में होता है। यह भौतिकी की एक प्रमुख और महत्वपूर्ण विधि है, जो समझने में समय लगाती है, लेकिन जब हम इसे समझ जाते हैं तो इसका उपयोग हमें विज्ञान और तकनीक के क्षेत्र में समस्याओं का समाधान करने में सहायक होता है।
Thermodynamics Notes PDF Class 11 for NEET
The detailed notes in the PDF form on the chapter thermodynamics is given below for the convenience of students. The notes contains all the minute details and specifications required to master this chapter keeping in mind the NEET syllabus. The PDF notes have been created by the expert Chemistry Faculty of Adda 247 himself. These notes will prove to be a savior for students who struggle to understand the complex concepts of thermodynamics. The notes cover previous year questions with solutions to help students in understanding how to approach numerical problem of this chapter. The detailed notes on thermodynamics (that also includes detailed explanation of the 2nd law of thermodynamics) is given below.
Download Thermodynamics Chemistry NEET Notes PDF
Second law of Thermodynamics Examples
Question : To remove 500 J of heat from a low-temperature reservoir, a heat pump performs 400 J of effort. How much heat is transported to a hotter reservoir?
Solution: W = 400 J
QC = 500 J
QH = W + QC
QH = 400 J + 500 J
QH = 900 J
Thermodynamics Chemistry NEET Lecture
The detailed lecture video on thermodynamics for the NEET exam is given below. The video has explained all the relevant topics of thermodynamics chemistry for NEET aspirants in a simple way. Students are advised to go through this video lecture to get complete conceptual clarity of this topic.




 JEE Mains Result 2025 Session 2 Live: Sc...
JEE Mains Result 2025 Session 2 Live: Sc...
 UP, MP, CBSE Board Result 2025 Live Upda...
UP, MP, CBSE Board Result 2025 Live Upda...
 [Live] CUET UG Date Sheet 2025 @cuet.nta...
[Live] CUET UG Date Sheet 2025 @cuet.nta...










