In our daily lives, We all widely use Sodium Chloride in our household and various purposes. But few of them know that the basic salt that we use in our food for taste is sodium chloride. Sodium chloride is also known as table salt, common salt, or halite (the mineral form of common salt).
The chemical formula for sodium chloride is NaCl, which represents a 1:1 ratio of sodium and chloride ions. The applications of sodium chloride or Nacl are extensive including in the hospital, food industry, and so on. In this article, we are going to learn deeply about the sodium chloride formula, its properties, and its applications.
What is Sodium chloride NaCl?
Sodium chloride is a water-soluble ionic compound composed of sodium cation and chloride anion. Sodium chloride has a molecular weight of 58.44g/mol and is a white crystalline solid. It is found as rock salt in coastal seas and oceans. NaCl is composed of between 1% and 5% seawater.
Sodium chloride is responsible for seawater salinity and the extracellular fluid of various multicellular organisms. Sodium is extensively used as a condiment and food preservative in household to industrial processes
Sodium chloride formula
The Chemical formula of Sodium Chloride is NaCl. Nacl is an inorganic compound, which is formed when the elements Na (sodium) and Cl (chloride) combine to form white, crystalline cubes. As the Sodium chloride Formula, This compound consists of sodium cation and chloride anion. In sodium chloride Structure, the ratio of sodium and chloride ions is 1:1.
Sodium chloride Structure
Sodium chloride or Nacl is an ionic substance. It is composed of sodium ions that have gained an electron and have become positively charged (Na+) and chloride ions that have lost an electron and have become negatively charged (Cl-). The structure of NaCl is as follows:
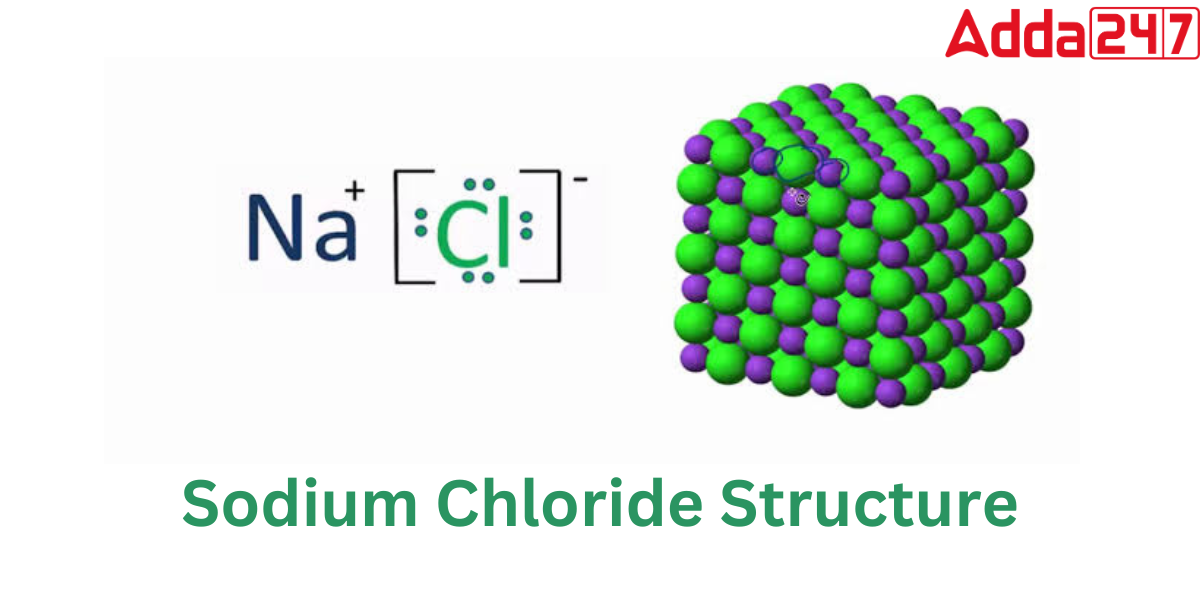
NaCl has a cubic crystal system and a face-centered cubic crystalline structure. A face-centered cubic unit cell of sodium chloride has four cations and four anions. Because each unit cell of a face-centered cubic structure has four atoms or ions, a unit cell of NaCl comprises four NaCl units.
Occurrence of Sodium Chloride
In nature, sodium chloride, sometimes known as salt, is widely present. Salt is a significant component of the dissolved elements in seawater. Sodium chloride is currently mass-produced via evaporation of seawater or brine from brine wells and salt lakes Mining the deposits yields sodium chloride, and running water through the deposits yields a brine solution. As a result, the salts dissolve and the solution is pumped out.
One of the primary methods for obtaining salt is the evaporation of seawater. Calcium sulfate, sodium sulfate, and other impurities are typically discovered in the crystals formed. To make pure crystals, dissolve the salts in a tiny amount of water and filter the solution.
Preparation of Sodium Chloride (NaCl)
Both chlorine and sodium combine to form sodium chloride, often known as table salt or common salt. The reaction for sodium chloride preparation is as follows:
2Na(s) + Cl2(g) → 2NaCl(s)
Properties of Sodium Chloride
Now Low at the key properties of the Sodium chloride
- Sodium chloride is an ionic substance that contains sodium and chloride ions in a 1:1 ratio.
- NaCl has a molecular weight of 58.44g/mol.
- It is easily soluble in water and only partially or completely soluble in other liquids.
- Sodium chloride has a pH of 7.
- They are white crystals with no odor but a distinct taste.
- NaCl is a good conductor of electricity in its aqueous condition because of the unrestricted movement of the ions,
- It has a melting point of 801 degrees Celsius and a boiling point of 1413 degrees Celsius.
Sodium chloride in water
Sodium chloride dissolves easily in water. When salt and water are mixed, the water molecules dissolve the Na and Cl atoms. The reaction occurs when water reacts with Sodium chloride as follows:
NaCl + H2O =NaOH + H2O + Cl2.
Sodium chloride (NaCl) is a compound composed of two ions, Na+ and Cl-, which are electrostatically attracted to one another and form a crystal. Water molecules are electrically neutral, yet their shape leads them to be polarized. Under the stronger forces caused by the water molecules, this characteristic causes the Na+ and Cl- ions to separate. It’s worth noting that the orientation of the water molecules alters when attracting a Na+ ion versus attracting a Cl- ion.
Sodium Chloride Uses
Sodium chloride, often known as common salt, has a wide range of applications, ranging from our kitchen to large-scale industries there are various use of Sodium Chloride or Nacl as follows
- It is widely used as a preservative and flavor enhancer in the food sector.
- It is used in cold countries to prevent ice from forming on roads, bridges, and other structures, which is essential for safe driving conditions.
- In order to soften water, sodium chloride is utilized. Calcium and magnesium ions can be found in hard water. Ion-exchange resins are used in commercial and household water-softening devices to extract the offending ions that cause hardness. The use of sodium chloride in the manufacture and regeneration of these resins.
- It is an essential raw ingredient in the industrial production of many compounds, including sodium carbonate and sodium hydrogen carbonate.
- In the pulp and paper business, salt is used to bleach wood pulp.
- It is also used to make sodium chlorate, which is then mixed with sulfuric acid and water to make chlorine dioxide, an excellent oxygen-based bleaching agent.
- Sodium chloride is used in the manufacture of sodium carbonate and calcium chloride. After that, sodium carbonate is used to manufacture glass, sodium bicarbonate, colors, and a range of other compounds.
What is sodium chloride used for in hospitals?
Sodium is an electrolyte that maintains your body’s water content. A sodium chloride injection is used to replace lost water and salt in your body as a result of specific diseases (for example, hyponatremia or low salt syndrome) .In hospitals, 0.9% salt Chloride Injection USP is used to restore extracellular fluids, cure metabolic alkalosis caused by fluid loss, and treat minor salt deficiency.
Sodium chloride Side effects
Sodium chloride is a medication used to cure or prevent sodium loss caused by dehydration, excessive sweating, or other factors. If you have ever had an allergic reaction to chloride or if you have excessive sodium levels in your blood, you should reduce your intake. Excess sodium chloride consumption has a number of negative side effects.
- High blood pressure
- Fast heart Beat
- kidney disease
- Congestive heart failure
- Liver disease (particularly cirrhosis); a buildup of fluid around your lungs.
- Fluid retention (especially swelling in your ankles or feet)
Although salt and sodium are not the same, salt contains 40% sodium and accounts for the majority of our sodium consumption and one teaspoon of salt contains approximately 2,300 milligrams (mg) of sodium, Salt is used by many businesses and restaurants to preserve, season, and taste their food.









 CBSE Admit Card 2026 for Private & R...
CBSE Admit Card 2026 for Private & R...
 AILET 2026 AIR 1: Check Full Toppers Lis...
AILET 2026 AIR 1: Check Full Toppers Lis...
 AILET Result 2026 OUT, How to Download S...
AILET Result 2026 OUT, How to Download S...














