Table of Contents
Solution is an important chapter for the class 12 science stream students and class 12 solution notes are valuable in the preparation of this chapter both for board as well as competitive exams. Every day in our lives, we encounter many solutions around us. To help students understand the phenomena around us, students are made to study this concept in their 12th class. The solution class 12 notes for NEET has also been provided for better understanding.
This chapter is one of the most crucial chapters from the examination point of view. So, one must master this chapter by heart to score good marks in the exam. Every year, around 2 questions are asked directly from the solutions concept.
Solution Class 12 Notes
The class 12 chemistry chapter solutions notes has been provided in this article to aid students in their exam preparation for both board as well as competitive exams like NEET. The solution chapter features at chapter number 2 in the chemistry text book of Class 12 NCERT. The chapter on solutions deals with different concepts related to solutions like osmotic pressure, Van’t Hoff factor, Henry’s Law, Raoult’s Law, etc.
Chemistry Class 12 Chapter 2 of Chemistry, i.e., Solution, has a good weightage in both board and NEET exam. It is also a highly scoring chapter if prepared diligently. All topics and subtopics pertaining to solid, liquid, and gaseous solutions are covered in this chapter. For this purpose, our expert faculty for NEET chemistry at Adda 247 have specially created a detailed notes covering all the minute concepts related to this chapter.
Solution
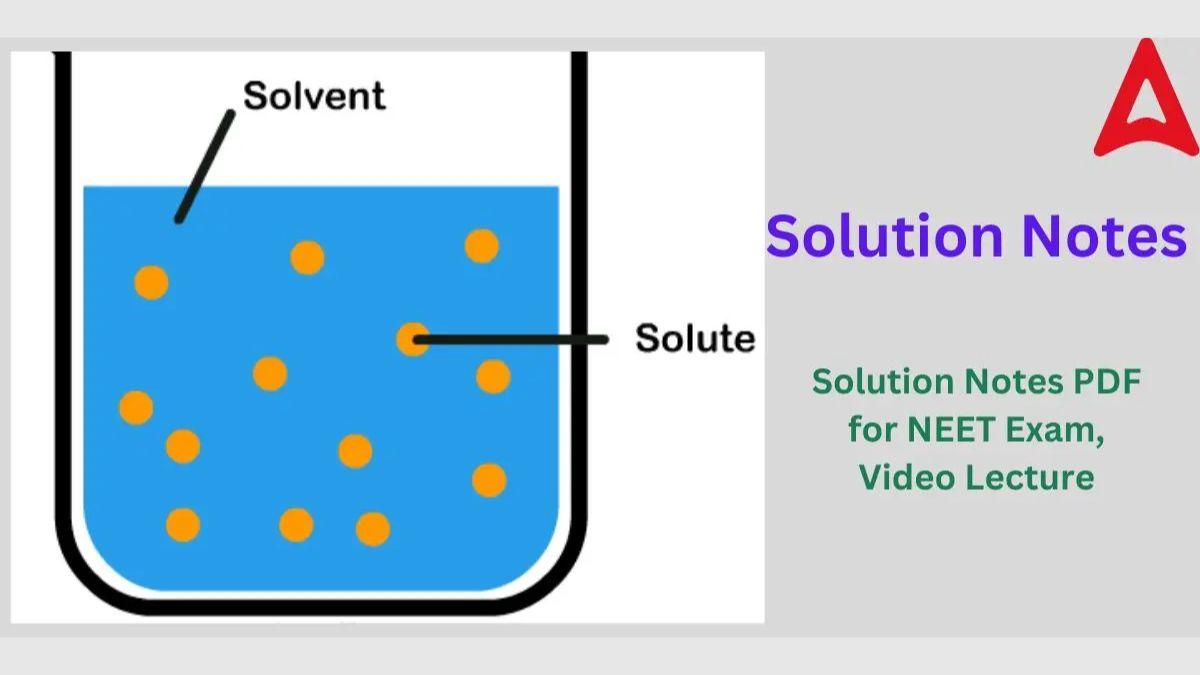
Homogeneous mixtures of two or more components are called solutions. A homogeneous mixture has a consistent composition and set of attributes throughout. A solution is made up of two components: Solute and Solvent
- Solvent: The component that makes up the majority of a solution is called the solvent, and it controls the physical condition of the mixture.
- Solute: Solute is another element of a solution that is present in lesser amounts.
Solution Types
There are 3 types of solutions. The name of these 3 types along with their details is tabulated hereunder.
| Solution Type | Solute | Solvent | Common Examples |
| Gaseous Solutions |
|
Gas |
|
| Liquid Solutions |
|
Liquid |
|
| Solid Solutions |
|
Solid |
|
Solution Class 12 Notes NCERT
NCERT is the bible to follow for the preparation of all the competitive exams like NEET. In NEET exam, the chemistry section has almost 70 percent to 80 percent of direct questions from the NCERT text book. This observation is sufficient to understand the importance of the NCERT notes for mastering this chapter. Some of the important NCERT notes for the Solution chapter of class 12th have been given below.
- 1. A homogenous combination of two or nine or more chemically inert components is referred to as a solution.
Usually, filtering, settling, or centrifuging cannot be used to separate the components of a solution. - There are three different types of solutions: solid, liquid, and gaseous.
- The quantity of solute in a saturated solution per 100g of a solvent is known as solubility.
- A gas’s solubility in a liquid is influenced by (a) the gas’s and the liquid’s composition, (b) the system’s temperature, and (c) the gas’s pressure.
- Henry’s Law regulates how pressure affects a gas’s solubility in a liquid. It asserts that a gas’s partial pressure is closely correlated with its solubility in a liquid at a particular temperature. P is equal to KHX in mathematics, where KH is Henry’s Law constant, X is the mole percentage of gas in the solution, and P is the partial pressure of the gas.
- The pressure that a liquid’s vapor exerts in a closed container while it is in dynamic equilibrium with its liquid is known as the vapor pressure of the liquid.
- Raoults Law states that the mole fraction of the solvent ( XA) is directly proportional to the vapor pressure of a solution containing a non-volatile solute. The pure solvent’s vapor pressure, or P = P° XA or PΗ XA, is the proportionality constant.
- An ideal solution is one that satisfies Raoult’s Law at all concentrations and temperatures.
- If the solution’s vapor pressure is higher than what Raoult’s Law predicts, it exhibits positive departure from the law. In the event when the solution’s vapor pressure is less than what Raoult’s Law predicts, a negative deviation is evident.
- Colligative properties are those characteristics of a solution that are only dependent on the quantity, not the type, of solute particles present. These characteristics include (a) a relative decrease in vapor pressure, (b) an increase in boiling point, (c) a decrease in freezing point, and (d) an increase in osmotic pressure.
- Osmosis is the term used to describe the spontaneous movement of solvent molecules across a perfect semi-permeable membrane, separating a concentrated solution from a dilute solution.
- The pressure needed to be delivered to the solution side (higher concentration solution) in order to merely stop pure solvent from passing through a semipermeable membrane is known as the osmotic pressure (π). Mathematically, π = CRT= nB/V-RT, where n denotes the solution’s osmotic pressure and C denotes its concentration. The variables nB, V, R, and T represent the number of moles of solute, the volume of the solution in liters, the gas constant, and the temperature in Kelvin.
- Solutions that have the same osmotic pressure are said to be isotonic. Their molar concentrations are also the same. In solutions that are isotonic, π1 = π2. C1 equals C2 as well.
- The factor “T” has a value less than one in the event of an association, when the observed molar mass is greater than the normal. However, when dissociation occurs, the observed molar mass has a smaller value, meaning that the Van’t Hoff factor is greater than one.
- The Van’t Hoff factor, “i,” will be equal to one in the case of solutes that do not experience any association or dissociation in a solvent since the observed and normal molar masses will be the same.
Solution Class 12 Notes for NEET
Questions from the chemistry class 12 chapter of solution is asked every year in the NEET UG exam. The solution chapter is an important part of class 12 chemistry and an important sub-topic of the physical chemistry. Every year, some questions are guaranteed from the concept of solution in the NEET exam. SO preparing this chapter by heart can fetch students up to 12 crucial marks.
As every mark matter in the NEET exam, so mastering this chapter will only increase the prospect of selection. The NEET exam demand students to have a very strong conceptual clarity, so by preparing the expert-made notes, students will be able to match that level of preparation. That is why, we have provided students with the solution notes PDF for NEET exam.
Solution Chapter Notes PDF for NEET
The detailed notes for the solution chapter in PDF is given below.
Download Solution Class 12 Notes PDF for NEET Part 1
Download Solution Class 12 Notes PDF for NEET Part 2
Solution Class 12 Video Lectures for NEET
The expert faculty at Adda247 have created two detailed video lectures on the Solution chapter for the NEET exam. The videos cover all the minute details and important previous year questions with solutions. By going through these videos, students will have a full conceptual clarity for the NEET exam.




 NEET Passing Marks Out of 720, Check NEE...
NEET Passing Marks Out of 720, Check NEE...
 NEET Total Marks- Check NEET Marks Distr...
NEET Total Marks- Check NEET Marks Distr...
 NEET Doubt Solving App, Free Instant Dou...
NEET Doubt Solving App, Free Instant Dou...








