Table of Contents
Titration is one of the most important concepts in the realm of chemical analysis. We can easily calculate the titration value using the titration formula. Titration is a widely used analytical method that is utilized to quantify the concentration of a substance in a solution. This entails introducing a specified quantity of titrant to the analyte solution until a reaction takes place.
In this article, we will learn about the Titration formula in detail along with other aspects of titration. Before knowing about the formula for titration, let us understand what does Titration means.
Titration
Titration is a commonly utilized analytical method in chemistry that enables the measurement of the concentration of a specific substance in a solution. It includes adding a known concentration solution, known as the titrant, in a controlled manner to the analyte solution until the reaction between them is finished. The concentration of the analyte can be determined by measuring the volume of the titrant needed to reach the reaction’s endpoint with precision.
Titration Definition
Titration, also known as titrimetry, is an essential qualitative analytical method in chemistry used to measure the concentration of a substance in a mixture. It is sometimes also referred to as Volumetric Analysis. Titration is a laboratory method that determines the concentration of a mystery solution by using a solution of known concentration. Titration is a crucial method for establishing the concentration of a solution of unknown composition in a broad sense.
Titration Formula
The titration formula is expressed as:
% Acid = (N × V × Equivalent Weight) × 100 / (W × 1000)
A titration is a technique employed to find out the amount of one solution needed to react accurately with a given volume of another solution. Titration involves different kinds of reactions, such as redox and precipitation reactions, that are distinct from acid-base reactions. The goal of a titration is to determine the precise amount of one solution required to fully react with a specified volume of another solution. The representation of formula is given below:
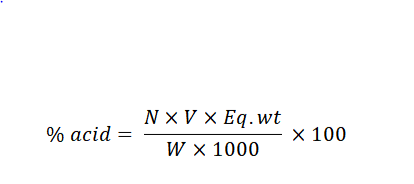
Where,
N = normality of the titrant
V = volume of the titrant
Eq. wt = equivalent weight of the acid
W = mass of the sample
1000 = conversion factor for milligrams to grams
Alternate Titration Formula
Apart from the above formula, there is another formula used for the titration concept which is based on stoichiometry. The alternate titration formula is give hereunder.
C1V1 = C2V2
where,
C1 = Concentration of the analyte (initial substance) in mol/L
V1 = Volume of the analyte solution used in the titration in litres
C2 = Concentration of the titrant (known solution) in mol/L
V2 = Volume of the titrant solution used to reach the endpoint in litres
This equation relies on stoichiometry, using the mole ratio of the analyte to the titrant to determine the analyte’s concentration. It enables the calculation of the unidentified analyte concentration by comparing it to the known titrant concentration and volume.
Types of Titration
There is a variety of titration types, with each one designed to focus on specific chemical reactions or substances of interest. Below are some of the commonly utilized types of titration and their respective uses that are covered.
Acid-Base Titration
An acid-base titration is the process of neutralizing either an acid or a base with a standard solution of the opposite type. This is one of the most frequently performed types of titration. An appropriate marker, like phenolphthalein or bromothymol blue, is included in the analyte solution to show the endpoint. Acid-base titration is a common method employed in industries such as pharmaceuticals, environmental monitoring, and food and beverage to measure the concentration of acids, bases, or salts.
Complexometric Titration
Complexometric titration is used for calculating the amount of metal ions present in a solution. It includes the creation of a complex between the metal ion and a complexing agent (ligand). A chelating indicator is the term for the indicator utilized in complexometric titration.
EDTA is a frequently utilized chelating agent. This method of titration is used for analyzing the hardness of water, identifying metal impurities in medicines, and quantifying the calcium and magnesium levels in soil samples.
Volumetric Titration
Volumetric titration, or simple titration, is the process of determining the volume of titrant solution needed to reach the endpoint. This form of titration is commonly used for regular analysis in labs, where the amount of the substance being analyzed is found by measuring the volume of the substance being added. Volumetric titration often includes acid-base, redox, and precipitation methods.
Redox Titration
Redox titration is characterized by redox reactions occurring between the analyte and the titrant. The endpoint is indicated by a shift in color or the formation/dissipation of a precipitate. Popular redox titrations involve finding the amount of oxidizing or reducing substances, like measuring vitamin C levels or determining the iron concentration in a sample. Redox titrations are commonly employed in pharmaceutical, environmental, and industrial testing.
Precipitation Titration
The endpoint of the reaction in precipitation titration is indicated by the formation of a precipitate. A common method to gauge halides (chloride, bromide, iodide) in a sample is by titrating with a solution of silver nitrate. The presence of a white solid (known as silver halide) signifies the conclusion. Precipitation titration is utilized for the examination of sulphate, carbonate, and other anions in different sectors.
Titration Formula Calculation
Let’s thoroughly examine the specifics of titration calculations gradually.
1) Balanced Chemical equation
The initial step in titration calculations involves setting up the balanced chemical equation for the interaction of the analyte and the titrant. The equation shows how many moles are involved in the reaction by giving the stoichiometric relationship between the two substances.
2) Molar Ratio
The molar ratio between the analyte and the titrant is provided by the balanced chemical equation. This proportion shows the amount of titrant moles needed to react with one mole of the analyte. Converting between the volume of the titrant used and the number of moles of the analyte is essential.
3) Volume of Titrant Used
The measure of the volume of the titrant solution used in the titration is taken in the course of the experiment. The amount is usually stated in millilitres (mL) or litres (L) in this recording.
4) Concentration of Titrant
The molarity or mol/L of the titrant solution is either known or can be calibrated. This data is essential in calculating the amount of moles of the titrant utilized in the reaction.
5) Normality of Titrant
In some titrations, normality (N) of the titrant is utilized instead of molarity. The measure of normality is the quantity of solute equivalents present in one liter of solution. The calculation of normality involves the multiplication of the titrant’s molarity by the solute’s equivalents per mole. Accurate titration calculations necessitate this information.
6) Volume of Analyte
Measurement of the volume of the analyte solution utilized during titration is also conducted. Just like the amount of titrant used, it is typically measured in milliliters (mL) or liters (L).
Titration Calculation Steps
The steps for titration calculations are determined by the information provided above.
- If needed, change the amount of titrant used from milliliters to liters.
- Determine the moles of the titrant by using the concentration of the titrant and the volume that has been converted.
- The molar ratio between the titrant and the analyte is determined by the balanced chemical equation.
- Determine the amount of moles of the analyte by utilizing the molar ratio.
- Translate the quantity of analyte from moles to the required measurements, like grams or percentages, according to the analysis criteria.
Titration Formula: Procedure
The titration procedure begins with the preparation of a standardized solution known as a titrant or titrator, which has a specific volume and concentration. This reagent is then permitted to react with the sample until a conclusion point or equivalence point is reached. At this point in time, the concentration of the analyte can be estimated by determining the amount of titrant used.
Titration is essentially a method used to determine the concentration of an unknown solution by measuring it stoichiometrically. Regarding the procedural steps, a specific amount of the substance being analyzed is added to either a beaker or an Erlenmeyer flask. A small amount of the titrant, usually with an indicator such as phenolphthalein, is placed below a calibrated burette or chemical pipetting syringe.
Slowly, small amounts of the titrant are added to the analyte solution along with the indicator. This procedure goes on until the change in color of the indicator indicates that the titrant has reached its saturation point. At this point, it signifies the end of the titration process. Basically, the amount of titrant in the reaction is equal to the amount of analyte in the solution.
Titration Preparation Techniques
It is crucial that both the titrant and the analyte are present in liquid form, usually as solutions. Substances solid substances are frequently dissolved with solvents such as glacial acetic acid or ethanol. Moreover, when analytes are highly concentrated, dilution is frequently carried out to improve accuracy. During numerous titrations not involving acids or bases, it is important to keep the pH stable by adding a buffer solution to the titration container.
In some instances in the reaction container, an additional masking solution can be added to counteract the effects of unwanted ions. In order to speed up specific redox reactions, it might be required to warm the sample solution and perform the titration while it stays heated.
Titration Formula: Chemical Analysis
Chemical analysis is the process of identifying and measuring the chemical components present in a substance. There are two main types of categorization for this form of analysis.
- Qualitative Analysis: Its main focus is to recognize the many chemical species found in a compound.
- Quantitative Analysis: It entails quantifying the amounts of chemical substances found in a compound.
Titration is a method of quantitative chemical analysis used to determine the concentration of a particular chemical in a solution. Different techniques can be used to establish the endpoint of a titration, such as:
- Gravimetric Analysis
- Volumetric Analysis
- Combustion
- Spectroscopy
A solid understanding of basic principles like the mole concept for balanced chemical equations and equivalence concepts for unbalanced chemical reactions is necessary to accurately find an unknown concentration during titration.
Applications of Titration Formula
Listed below are a few illustrations showcasing the practical use of the titration formula:
- Measuring the strength of an acidic or alkaline solution
- Measuring the amount of salt concentration
- Identifying the amount of a metal ion in a solution
- Identifying the amount of a substance found in an organic compound.
- Assessing the quality of a material
- Measuring the level of moisture in a material
Titration Formula: Important Points
Some of the important points regarding the Titration and its formula is given hereunder.
- Titration is a method in chemistry employed for measuring the concentration of a mysterious substance by utilizing a known concentration or titration formula.
- The typical equation for titration is MA multiplied by VA equals MB multiplied by VB.
- Four types of titrations include redox titration, Argentometric titration, complexation titration, and acid-base titration.
- Titration is a method that provides quantitative results. In this technique, the known concentration of an acid/base can be used to neutralize the unknown concentration of an acid/base.
Titration Formula Practice Questions
Example: Determine the titratable acidity, represented as the citric acid percentage, by using 17.5 mL of 0.085N NaOH to titrate a 15 mL juice sample (Molecular Weight = 192; Equivalent Weight = 64)
Solution: We can determine the titratable acidity by using the titration formula
Given, Molecular Weight = 64
Equivalent weight = 192
Volume = 17.5 mL
Normality = 0.085
Mass of the sample = 15 mL
Using the titration formula: % Acid = (N × V × Equivalent Weight) × 100 / (W × 1000) and putting the values, we get
% of acid = 0.085 × 17.5 × 64 / 15 × 10
% acid = 0.635%
Example: In a titration of sulfuric acid against sodium hydroxide, 32.20 mL of 0.250 M NaOH is required to neutralize 26.60 mL of H2SO4. Calculate the molarity of the sulfuric acid.
Step 1: List the known values and plan the problem.
Known
- molarity NaOH = 0.250 M
- volume NaOH = 32.20 mL
- volume H2SO4 = 26.60 mL
Unkonwn
- molarity H2SO4 = ?
First determine the moles of NaOH in the reaction. From the mole ratio, calculate the moles of H2SO4 that reacted. Finally, divide the moles H2SO4 by its volume to get the molarity.
Step 2: Solve.
The volume of H2SO4 required is smaller than the volume of NaOH because of the two hydrogen ions contributed by each molecule.
| Related Articles | |
| Mole Concept | Redox Reaction |
| Aluminium Fluoride Formula | Hydrofluoric Acid Formula |
| Theoretical Yield Formula | Molality Formula |





 CUET Chemistry Syllabus 2025, Download O...
CUET Chemistry Syllabus 2025, Download O...
 ICSE Class 10 Chemistry 30 Day Preparati...
ICSE Class 10 Chemistry 30 Day Preparati...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...










