Table of Contents
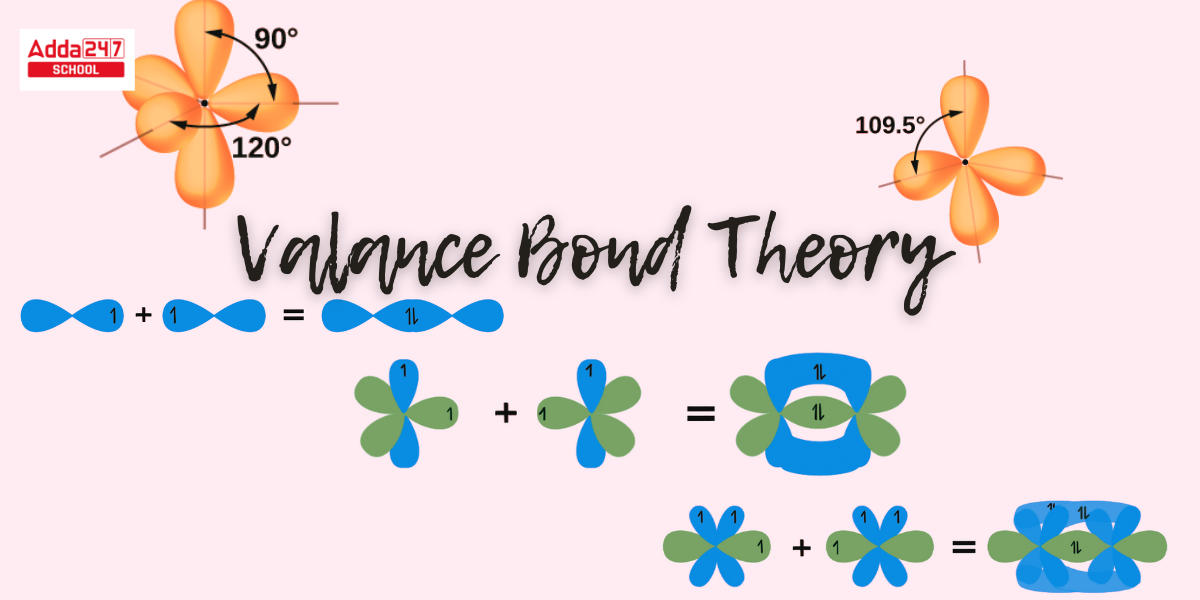
Valance Bond Theory: Valence bond theory (VBT) argues that all bonds in coordination molecules are localized bonds produced by an electron donation from each atom. The valence bond theory describes how electrons occupy an atom’s atomic orbitals inside a molecule. It also states that the nucleus of one molecule is drawn to the electrons of another. In some circumstances, VBT is also an erroneous inference since many atoms link via delocalized electrons. The VBT theory of molecular oxygen predicts that there are no unpaired electrons. Despite the fact that Valance bond theory does an excellent job of qualitatively explaining the forms of covalent compounds.
Valance Bond Theory
What is the Valance Bond Theory? The Valance Bond Theory was created to use quantum physics to demonstrate the concept of chemical bonding. VBT represents a molecule’s electrical structure. In accordance with this idea, electrons fill an atom’s atomic orbitals within a molecule. The Valance Bond Theory holds that electrons cover the atomic orbitals of individual atoms inside a molecule and that electrons from one atom stick to the nucleus of another. This attraction rises as the atoms go closer until they reach a point where the electron density causes repulsion among the two atoms. This electron density at the shortest distance connecting the two atoms acquires the lowest potential energy and can be thought to be what holds the atoms together in chemical bonding.
Valance Bond Theory Background
Valence bond structures were put forward by G.N. Lewis in 1916, based on the notion that chemical bonds are formed by two sharing electrons that link together. Valence bond theory employs Lewis structures.
Quantum mechanics was utilized to characterize bonding properties in the Heitler-London theory of 1927. This hypothesis explains the creation of chemical bonds between hydrogen atoms in the H2 molecule by using Schrödinger’s wave equation to combine the wave functions of the two hydrogen atoms.
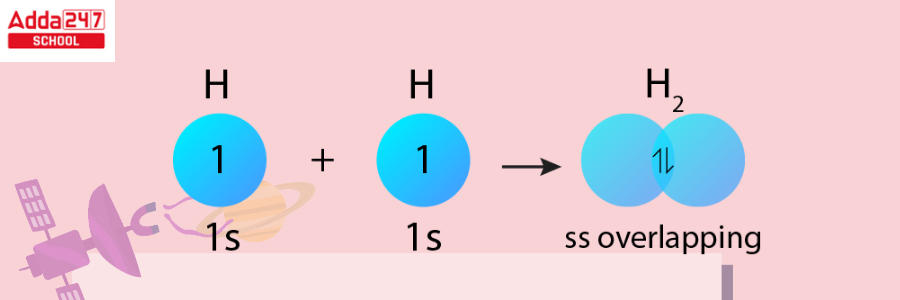
In 1928, Linus Pauling introduced the valence bond theory, which combined Lewis’s pair bonding concept with the Heitler-London hypothesis. The valence bond hypothesis was developed to shed light on resonance and orbital hybridization. Pauling published “On the Nature of the Chemical Bond,” a dissertation on valence bond theory, in 1931.
Initially, computer programmers used molecular orbital theory to depict chemical bonding, but valence bond theory concepts have been accessible since the 1980s. Today’s versions of these theories compete with one another in terms of adequately understanding real action.
Valance Bond Theory Important Facts
The valence bond theory proposes the following facts that you must know.
- Only when the partially filled orbitals of two atoms overlap do covalent bonds form.
- As a consequence of this overlapping, the electron density in the area between the two bonded atoms increases, boosting the stability of the resulting molecule.
- Each overlapping atomic orbital must have an unpaired electron with a polarity opposite to its own.
- The energies of the overlapping atomic orbitals should be substantially identical.
- Electron spins are neutralized, and electrons pair collectively on atomic orbitals that overlap.
- When atomic orbitals overlap, the electron density between the two nuclei expands, and thus the pulling between the two nuclei of bound atoms diminishes.
- A bond forms at an equilibrium range when the entire system has the least potential energy and the most stability.
- The extent of orbital overlap between two atoms impacts the strength of a covalent connection. The greater the overlap between atomic orbitals, the more energy is released and the bond established.
- The number of covalent bonds generated by an atom of an element is equivalent to the number of electrons without pairings in the atom’s valence shell.
- Each atomic orbital (save the s-orbital) has a distinct direction in space. As a result, covalent bonds generated by atomic orbital overlap have directional properties.
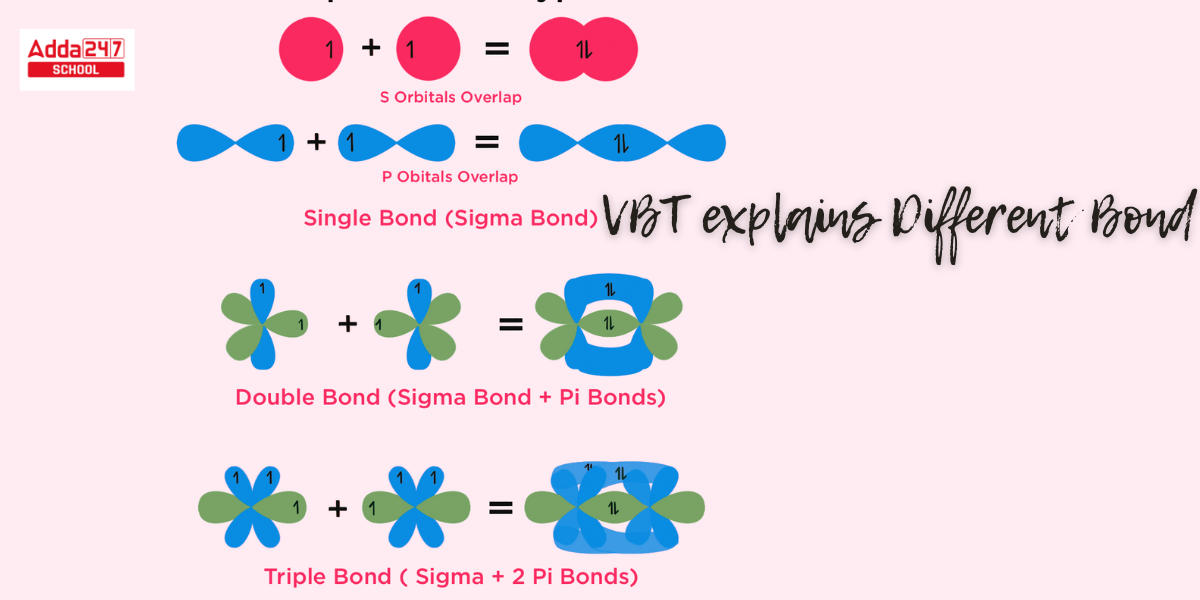
- The configuration of a molecule is determined by the directional alignment of overlapping orbitals; the s orbital is spherically symmetric and overlaps in all directions.
- To reduce electronic repulsion and maximize uniformity, orbitals are oriented so that they are far away from each other.
- Since p, d, and f orbitals have distinct spatial orientations, the shape of the orbital and bond angle are determined by the orientation of overlapped orbitals.
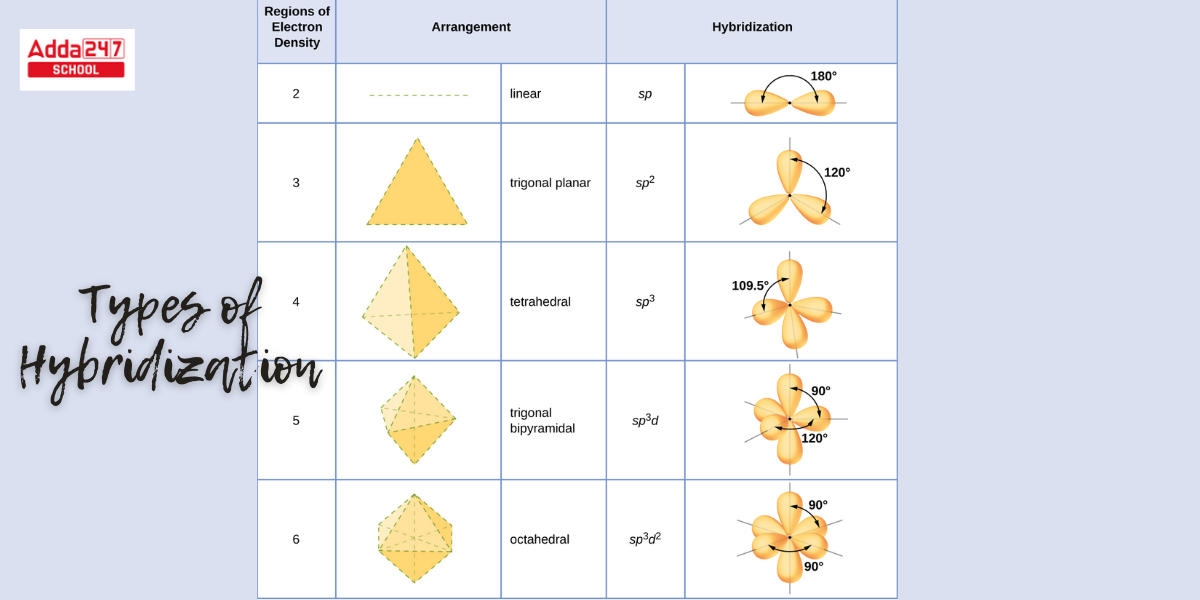
Types of Hybridization
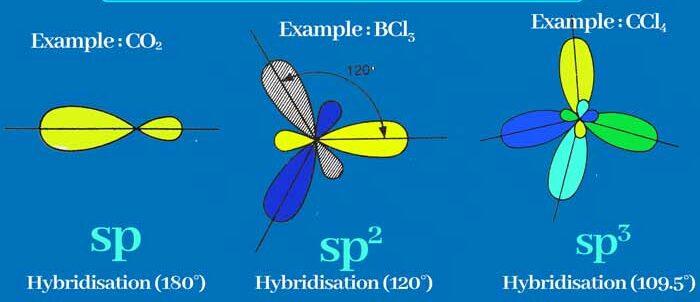
The concept of hybridization is that atomic orbitals merge to generate fresh hybridized orbitals, which influence molecule conformation and bonding properties. The valence bond hypothesis is additionally expanded by hybridization. To investigate this further, we used three different types of molecules (CO2, BCl3, CCl4) to demonstrate sp, sp2, and sp3 hybridization.
Under the influence of ligands, a metal atom or ion may utilize its (n-1)d, ns, np, or ns, np, nd orbitals for hybridization, yielding a set of analogous orbitals of specified geometry such as octahedral, tetrahedral, square planar, and so on. These hybrid orbitals may overlap with ligand orbitals capable of donating several electrons for bonding.
Applications of Valance Bond Theory
The Valence bond theory is significant because it aids in comprehending the concept of molecule bonding.
- The Valence bond theory presents few principles in chemical bonding, which are as follows:
Electron delocalization between the two nuclei.
The bond’s basic covalent nature.
Electrons have a shielding effect.
The presence of a covalent link with a partly ionic character.
The idea of resonance, as well as the relationship between resonance energy and molecule stability.
- The requirement of a maximum intersection, which indicates the establishment of the strongest conceivable bonds, is an important component of the Valence Bond theory. This notion is used to describe the formation of covalent bonds in numerous compounds.
For example, in the context of the F2 particle, the FF bond is formed by the intersection of the two F atoms’ pz orbitals, each of which contains an unpaired electron. As a consequence, the natures of the crossing orbitals in H2 hydrogen and F2 fluorine molecules vary, as do the binding strengths and bond lengths.
Valance Bond Theory Limitations
The Valence Bond Theory also has many flaws. Some of are are as follows:
- It fails to provide an explanation for carbon’s tetravalency.
- Valence bond theory only defines the creation of a covalent bond in which two bonding atoms exchange a pair of electrons. It does not, however, explain the creation of a coordinate covalent connection in which one of the connected atoms contributes both electrons.
- The energies of electrons are not discussed in this Valence Bond Theory.
- According to this theory, oxygen molecules must be diamagnetic. The two atoms in the oxygen molecule should have enclosed electronic shells, giving the molecule no unpaired electrons thereby rendering it diamagnetic. However, the molecule is discovered to be para-magnetic, with two unpaired electrons, in experiments. As a result, this theory falls short of explaining.
- The electrons are assumed to be localized to particular sites.
- Valence bond theory is unable to clarify bonding in electron-deficient compounds such as B2H6, where the central atom has fewer electrons paramagnetism of oxygen atoms than needed for an octet of electrons.


 NEET UG 2025: Is NEET Previous years Que...
NEET UG 2025: Is NEET Previous years Que...
 JEE Mains Session 2 Result 2025 OUT, Sco...
JEE Mains Session 2 Result 2025 OUT, Sco...
 TS Inter Results 2025 Date for TSBIE 1st...
TS Inter Results 2025 Date for TSBIE 1st...










