The number of atoms of one element bound to each atom of another in a molecule is depicted on a valency chart. Valence, or valency, is the property of an element that indicates how many additional atoms can join one of its atoms in a covalent connection. The valency chart gives valence electrons to each element in the periodic table, determining the number of possible bonds. In this post, we will look at what valency is, how to make a valency table or chart, how to calculate the valency of a molecule, and how to use a Valency Table, among other things.
Valency Table
A valency table (or valence table) in chemistry lists the valency (combining power) of different elements or ions. Valency represents the number of chemical bonds an atom of an element can form, typically determined by the number of electrons in its outermost shell (also called valence electrons).
For example:
| Element | Symbol | Valency |
|---|---|---|
| Hydrogen | H | 1 |
| Oxygen | O | 2 |
| Nitrogen | N | 3 |
| Carbon | C | 4 |
| Sodium | Na | 1 |
| Magnesium | Mg | 2 |
The valency of an atom helps predict how it will combine with other atoms to form chemical compounds. Elements often follow specific patterns based on their group in the periodic table.
Valency Table of First 30 Elements
In this Valency table of elements, we have mentioned the valency of 30 elements and their atomic number.
| Valency Table of Elements | ||
|
Atomic Number
|
Element | Valency Chart |
| 1 | Valency of Hydrogen | 1 |
| 2 | Valency of Helium | 0 |
| 3 | Valency of Lithium | 1 |
| 4 | Valency of Beryllium | 2 |
| 5 | Valency of Boron | 3 |
| 6 | Valency of Carbon | 4 |
| 7 | Valency of Nitrogen | 3 |
| 8 | Valency of Oxygen | 2 |
| 9 | Valency of Fluorine | 1 |
| 10 | Valency of Neon | 0 |
| 11 | Valency of Sodium (Na) | 1 |
| 12 | Valency of Magnesium (Mg) | 2 |
| 13 | Valency of Aluminium | 3 |
| 14 | Valency of Silicon | 4 |
| 15 | Valency of Phosphorus | 3 |
| 16 | Valency of Sulphur | 2 |
| 17 | Valency of Chlorine | 1 |
| 18 | Valency of Argon | 0 |
| 19 | Valency of Potassium (K) | 1 |
| 20 | Valency of Calcium | 2 |
| 21 | Valency of Scandium | 3 |
| 22 | Valency of Titanium | 4 |
| 23 | Valency of Vanadium | 5,4 |
| 24 | Valency of Chromium | 2 |
| 25 | Valency of Manganese | 7, 4, 2 |
| 26 | Valency of Iron (Fe) | 2, 3 |
| 27 | Valency of Cobalt | 3, 2 |
| 28 | Valency of Nickel | 2 |
| 29 | Valency of Copper (Cu) | 2, 1 |
| 30 | Valency of Zinc | 2 |
Valency Chart
A Valency Chart is intended for determining the amount of chemical bonds that a specific element can make with different elements. The valency table is a list of the element’s valencies.
Based on the element’s valence electrons, those are the outermost electrons in charge of bonding, it describes how an element can form bonds. There can be anywhere from one to eight valence electrons, depending on the type of element. It is vital to note that for the valency chart to be accurate, the elements must be organized in an atomic number order.
What does the term Valency imply?
The ability of an element to combine with other elements is telemetered as its valency.
- The term valency was coined in 1868 to describe both the broad potential of merging an element and the numerical magnitude of that capability.
- It is defined as the number of electrons lost or gained by an atom during a chemical reaction, or the number of electrons shared.
- Valence is characterized as the number of electrons since the bulk of bonds are formed by the exchange of valence electrons.
- In chemistry, valence electrons decide what valences are and what they represent.
Valency Concept with Examples
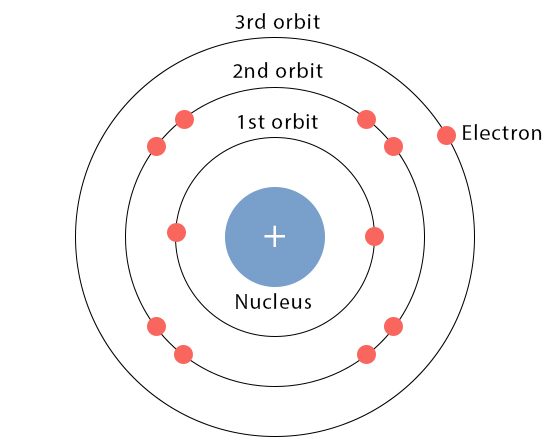
An atom’s valency is the number of additional atoms with which it can build a molecule. The letters K, L, M, N, and so on represent the various orbitals (shells) in which electrons are organized in atoms. Valence electrons are those located in an atom’s outermost orbit or shell. Valence electrons participate in every chemical process because their outermost orbit often contains more energy than electrons in other orbits.
Example –
- Hydrogen has a valency of one because it can interact with one other atom to form a molecule.
- We can see only 6 electron is present in the last shell of the oxygen molecule. Because it can form a molecule with two additional atoms, oxygen has a valency of two.
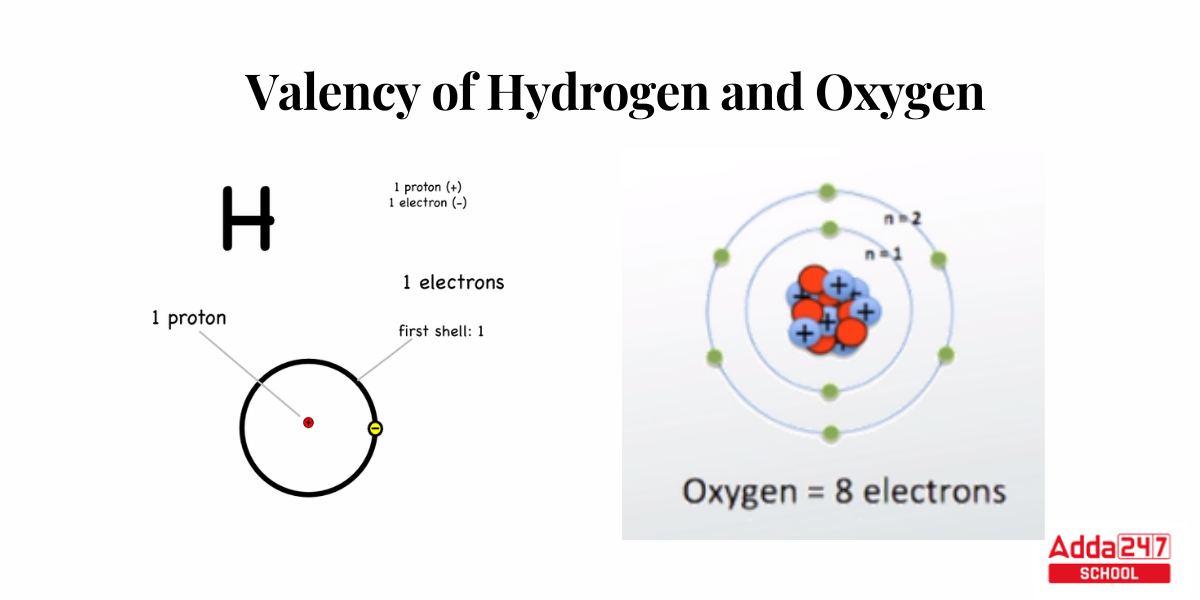
Bohr’s Theory
According to the Bohr-bury hypothesis, an atom’s outermost orbit can hold up to eight electrons. If the outermost orbit is filled, however, there is minimal to no chemical activity in the specific element. Their ability to merge is almost non-existent.
How to determine the valency of elements?
There are three primary methods for determining the valency of any element. The first one is the Octet method, knowing by chemical formula and periodic table.
- The Octet Method –
We can assess the valency of a molecule with octet rule. This principle states that atoms of an element or chemical have a proclivity to gain or lose electrons in the compound in which they are present, resulting in an increase or reduction in the amount of electrons in their outermost orbit. The maximum number of electrons in an atom’s outermost orbit is eight. If an atom has 8 electrons in its outermost shell, it is considered stable.
Chemical formula –
This approach the knowing formula the value from the chemical formula is built on the octet rule. It is feasible to discover the valencies of many transitional elements or radicals in a given molecule by observing how they interact chemically with other elements of known valency. In this case, the octet rule is used because the combining elements and radicals want to achieve a total of eight electrons in the outermost shell for them to become stable.
Periodic Table –
The periodic table of elements graphic is the most common tool to utilized to compute valency in this method. All of the metals in column 1 have a valency of +1, for example, hydrogen, lithium, sodium, and so on. Similarly, all of the elements in column 17 have valency 1, including fluorine, chlorine, and other elements. Columnorganizesises all of the noble gases. These chemicals are inert and have a valency of 0.
Valency Chart of Ions
Some ions also represent different valencies, some of the ions who have 1, 2,and 3 valencies respectively are mentioned below.
| Valency Chart of Ions | |
| Valency 1 | |
|
Name of Ions
|
Symbol |
| Sodium | Na+ |
| Potassium | K+ |
| Copper (I) | Cu+ |
| Hydrogen | H+ |
| Hydride | H– |
| Chloride | Cl– |
| Bromide | Br– |
| Ammonium | Al– |
| Hydroxide | OH– |
| Nitrate | NO3– |
| Oxalate | CH3COO– |
| Valency 2 | |
| Magnesium | Mg+2 |
| Calcium | Ca+2 |
| Copper (II) | Cu+2 |
| Iron (II) | Fe+2 |
| Zinc | Zn+2 |
| Oxide | O-2 |
| Sulphide | S-2 |
| Carbonate | CO2-2 |
| Sulphite | SO3-2 |
| Sulphate | SO4-2 |
| Valency 3 | |
| Aluminium | Al+3 |
| Iron (III) | Fe+3 |
| Nitride | N-3 |
| Phosphate | PO4-3 |
| Gold (III) | Au+3 |
| Titanium (III) | Ti+3 |
Valency Table of all 118 Elements
Valency Chart of All Elements, Representing the valency (also known as oxidation state) of all elements in a single chart can be quite challenging due to the large number of elements and their varying valencies depending on the compounds they form. Additionally, some elements can exhibit multiple oxidation states depending on the chemical environment.
list of the valency of all 118 elements. Valency is the combining power of an element, usually defined by the number of hydrogen atoms an element can combine with or displace in a compound.
- Hydrogen (H): 1
- Helium (He): 0
- Lithium (Li): 1
- Beryllium (Be): 2
- Boron (B): 3
- Carbon (C): 4
- Nitrogen (N): 3, 5
- Oxygen (O): 2
- Fluorine (F): 1
- Neon (Ne): 0
- Sodium (Na): 1
- Magnesium (Mg): 2
- Aluminium (Al): 3
- Silicon (Si): 4
- Phosphorus (P): 3, 5
- Sulfur (S): 2, 4, 6
- Chlorine (Cl): 1, 3, 5, 7
- Argon (Ar): 0
- Potassium (K): 1
- Calcium (Ca): 2
- Scandium (Sc): 3
- Titanium (Ti): 2, 3, 4
- Vanadium (V): 2, 3, 4, 5
- Chromium (Cr): 2, 3, 6
- Manganese (Mn): 2, 4, 7
- Iron (Fe): 2, 3
- Cobalt (Co): 2, 3
- Nickel (Ni): 2, 3
- Copper (Cu): 1, 2
- Zinc (Zn): 2
- Gallium (Ga): 3
- Germanium (Ge): 4
- Arsenic (As): 3, 5
- Selenium (Se): 2, 4, 6
- Bromine (Br): 1, 3, 5
- Krypton (Kr): 0, 2
- Rubidium (Rb): 1
- Strontium (Sr): 2
- Yttrium (Y): 3
- Zirconium (Zr): 4
- Niobium (Nb): 3, 5
- Molybdenum (Mo): 2, 3, 4, 5, 6
- Technetium (Tc): 7
- Ruthenium (Ru): 2, 3, 4, 6, 8
- Rhodium (Rh): 3
- Palladium (Pd): 2, 4
- Silver (Ag): 1
- Cadmium (Cd): 2
- Indium (In): 3
- Tin (Sn): 2, 4
- Antimony (Sb): 3, 5
- Tellurium (Te): 2, 4, 6
- Iodine (I): 1, 3, 5, 7
- Xenon (Xe): 0, 2, 4, 6, 8
- Cesium (Cs): 1
- Barium (Ba): 2
- Lanthanum (La): 3
- Cerium (Ce): 3, 4
- Praseodymium (Pr): 3
- Neodymium (Nd): 3
- Promethium (Pm): 3
- Samarium (Sm): 3
- Europium (Eu): 2, 3
- Gadolinium (Gd): 3
- Terbium (Tb): 3
- Dysprosium (Dy): 3
- Holmium (Ho): 3
- Erbium (Er): 3
- Thulium (Tm): 3
- Ytterbium (Yb): 2, 3
- Lutetium (Lu): 3
- Hafnium (Hf): 4
- Tantalum (Ta): 5
- Tungsten (W): 2, 3, 4, 5, 6
- Rhenium (Re): 2, 3, 4, 5, 6, 7
- Osmium (Os): 2, 3, 4, 6, 8
- Iridium (Ir): 3, 4
- Platinum (Pt): 2, 4
- Gold (Au): 1, 3
- Mercury (Hg): 1, 2
- Thallium (Tl): 1, 3
- Lead (Pb): 2, 4
- Bismuth (Bi): 3, 5
- Polonium (Po): 2, 4, 6
- Astatine (At): 1, 3, 5, 7
- Radon (Rn): 0
- Francium (Fr): 1
- Radium (Ra): 2
- Actinium (Ac): 3
- Thorium (Th): 4
- Protactinium (Pa): 5
- Uranium (U): 3, 4, 5, 6
- Neptunium (Np): 3, 4, 5, 6, 7
- Plutonium (Pu): 3, 4, 5, 6, 7
- Americium (Am): 3, 4, 5, 6
- Curium (Cm): 3
- Berkelium (Bk): 3, 4
- Californium (Cf): 3
- Einsteinium (Es): 3
- Fermium (Fm): 3
- Mendelevium (Md): 2, 3
- Nobelium (No): 2, 3
- Lawrencium (Lr): 3
- Rutherfordium (Rf): 4
- Dubnium (Db): 5
- Seaborgium (Sg): 6
- Bohrium (Bh): 7
- Hassium (Hs): 8
- Meitnerium (Mt): 1 (predicted)
- Darmstadtium (Ds): 1 (predicted)
- Roentgenium (Rg): 1 (predicted)
- Copernicium (Cn): 2 (predicted)
- Nihonium (Nh): 1 (predicted)
- Flerovium (Fl): 2 (predicted)
- Moscovium (Mc): 1 (predicted)
- Livermorium (Lv): 2 (predicted)
- Tennessine (Ts): 1 (predicted)
- Oganesson (Og): 0 (predicted)
The valency of the elements can vary based on their chemical environment. Elements like sulfur and phosphorus show multiple valencies. Students can create valencies of all 118 elements pdf with the help of this article easily.
Valency and Oxidation State
Valence electrons are electrons present in the outermost shell. The primary distinction between valency and oxidation state is that valency is the number of electrons present in an atom’s valence shell, whereas oxidation state is the ability of a bit to acquire or lose electrons within an atom’s compound.
- The number of bonds is indicated by valency; the number of bonds is not indicated by oxidation state.
- A charge does not show valency; however, an electrical charge does indicate oxidation status.
- The periodic table determines the valency of s-block and p-block elements as the number of valence electrons or eight minus the count of valence electrons.
- The valency levels of d-block and f-block elements differ depending on whether they have cation or anion orbitals.
- The valencies of the d and f-block elements are typically 2 and 3.
Uses of Valency Chart
- Valency is a measure of an element’s combining capability. The number of atoms of an element that can combine to create a molecule is defined as its valency.
- Another major application of element valency is determining or deducing compound formulas. If we understand the valency of an element, we may readily develop formulas for its compounds.
- The valency of an element can be determined by examining the electron configurations of the atoms. An atom’s valency is the number of electrons it can lose or reverse to create a molecule.
Valency Table for Class 9 and 10
A valency table, also known as a valence table or valence electron table, is a chart that provides information about the valence electrons of elements in the periodic table. Valence electrons are the electrons located in the outermost energy level (shell) of an atom and are crucial in determining an element’s chemical behaviour, especially in forming bonds with other elements to create compounds.
The valency table typically lists elements in the periodic table along with their corresponding valence electrons. It helps chemists and students quickly understand how elements might bond with each other to form molecules. The valence electrons of an element can often be determined by its position in the periodic table. Here’s a simplified example of a valency table based on the first three periods of the periodic table:
| Element | Valence Electrons | Possible Valency |
|---|---|---|
| H | 1 | 1 |
| He | 2 | 0 |
| Li | 1 | 1 |
| Be | 2 | 2 |
| B | 3 | 3 |
| C | 4 | 4 |
| N | 5 | 3, 5 |
| O | 6 | 2 |
| F | 7 | 1 |
| Ne | 8 | 0 |
| Na | 1 | 1 |
| Mg | 2 | 2 |
In this table, the “Valence Electrons” column indicates the number of valence electrons for each element, while the “Possible Valency” column lists the potential valency values based on the element’s valence electrons. The valency values indicate the number of bonds an element can form when combining with other elements.
Keep in mind that the valency table becomes more complex as you move further down the periodic table due to the different energy levels and orbital structures of the elements. Additionally, transition metals and elements in higher periods may have multiple possible valencies due to their more complex electron configurations.








 Chemistry Investigatory Project Class 12...
Chemistry Investigatory Project Class 12...
 CBSE Class 12 Chemistry Viva Questions w...
CBSE Class 12 Chemistry Viva Questions w...
 CUET Chemistry Syllabus 2026, Download O...
CUET Chemistry Syllabus 2026, Download O...














