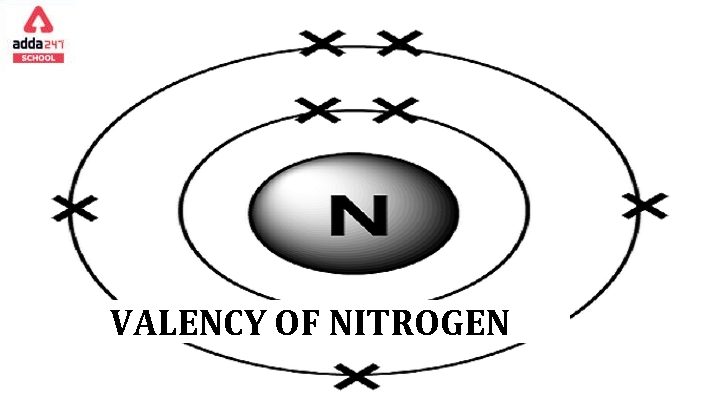
Valency of Nitrogen
The valency of an element refers to its combining capacity with other elements to form compounds. Nitrogen (N) typically exhibits a valency of Nitrogen is 3 and 5.
Valency of 3: Nitrogen has three valence electrons in its outermost energy level. It can share these electrons with three other atoms or groups to form covalent compounds. Ammonia (NH₃) is an example where nitrogen shares its three electrons with three hydrogen atoms.
Valency of 5: Nitrogen can also gain or lose three electrons to achieve a stable electron configuration like the nearest noble gas, which in this case is neon. By doing so, it can form compounds with a valency of 5, known as nitrate compounds. An example is nitrate ion (NO₃⁻), where nitrogen has a +5 oxidation state and has formed three covalent bonds with oxygen atoms.
It’s important to note that the concept of valency is often used to explain the combining capacity of elements in a simplistic way, especially for teaching purposes. In reality, the behavior of elements in chemical reactions is more complex and can involve different types of bonding and electron sharing.
Nitrogen valency
Nitrogen valency is 2 and 5. Nitrogen, which is situated at the top of Group 15 on the periodic table, has either 3 or 5 valence electrons. Because it can bond in the outer 2p and 2s orbitals, it can have 3 or 5 valence electrons. Molecular nitrogen (N2) is a colorless and odorless gas that is not reactive at typical temperatures and pressures.
What is The Valency of Nitrogen
what is the valency of nitrogen? The answer to this question is given below. Nitrogen can have various valencies, they are:
- It is -3 in NH3.
- It is 0 in NO.
- It is +1 in N2
- It is +2 in NO.
- It is +3 in N2O5.
- It is +4 in NO
- It is +5 in N2O5.
Valency of Elements- Valency Table Class 9
The valency of the first 30 elements of the periodic table is given below.
| Element | Atomic Number | Valency |
| Valency of Hydrogen | 1 | 1 |
| Valency of Helium | 2 | 0 |
| Valency of Lithium | 3 | 1 |
| Valency of Beryllium | 4 | 2 |
| Valency of Boron | 5 | 3 |
| Valency of Carbon | 6 | 4 |
| Valency of Nitrogen | 7 | 3 |
| Valency of Oxygen | 8 | 2 |
| Valency of Fluorine | 9 | 1 |
| Valency of Neon | 10 | 0 |
| Valency of Sodium (Na) | 11 | 1 |
| Valency of Magnesium (Mg) | 12 | 2 |
| Valency of Aluminium | 13 | 3 |
| Valency of Silicon | 14 | 4 |
| Valency of Phosphorus | 15 | 3 |
| Valency of Sulphur | 16 | 2 |
| Valency of Chlorine | 17 | 1 |
| Valency of Argon | 18 | 0 |
| Valency of Potassium (K) | 19 | 1 |
| Valency of Calcium | 20 | 2 |
| Valency of Scandium | 21 | 3 |
| Valency of Titanium | 22 | 4 |
| Valency of Vanadium | 23 | 5,4 |
| Valency of Chromium | 24 | 2 |
| Valency of Manganese | 25 | 7, 4, 2 |
| Valency of Iron (Fe) | 26 | 2, 3 |
| Valency of Cobalt | 27 | 3, 2 |
| Valency of Nickel | 28 | 2 |
| Valency of Copper (Cu) | 29 | 2, 1 |
| Valency of Zinc | 30 | 2 |
Valency of Nitrogen is Positive and Negative
Nitrogen is found in nearly all proteins and plays a vital function in biochemical and commercial applications. Because of its propensity to make a triple bond with itself and other elements, nitrogen produces strong bonds. As a result, nitrogen molecules contain a lot of energy. Before the turn of the century, nothing was understood about nitrogen. Nitrogen is now widely utilised as a food preservative and fertiliser.
- Nitrogen is a non-metal element found in abundance in the atmosphere; nitrogen gas (N2) makes up 78.1 percent of the Earth’s atmospheric volume.
- By mass, it scarcely makes up 0.002% of the earth’s crust.
- Foods, explosives, toxins, and fertilisers all contain nitrogen compounds.
- Nitrogen is found in both DNA and neurotransmitters in the form of nitrogenous bases.
- It’s one of the most common industrial gases, and it’s available as both a gas and a liquid.
Discovery of Nitrogen Valency
At ambient temperature, nitrogen, which makes up around 78 percent of our atmosphere, is a colourless, odourless, tasteless, and chemically inert gas. It gets its name from the Greek words nitron and genes, which mean “to make soda.” During the 1500s and 1600s, scientists speculated that there was a third gas in the atmosphere, in addition to carbon dioxide and oxygen. It wasn’t until the 1700s that scientists were able to prove that there was another gas that took up mass in the Earth’s atmosphere.
- Daniel Rutherford (and others independently, such as Priestly and Cavendish) discovered it in 1772 when he was able to remove oxygen and carbon dioxide from a confined tube full of air.
- He demonstrated that there was residual gas that could not be burned, such as oxygen or carbon dioxide.
- While his experiment demonstrated the existence of nitrogen, other tests were taking place in London, where the substance was referred to be burnt or dephlogisticated air.
Nitrogen is one of the most abundant elements in humans and is more abundant than carbon or silicon in the known universe. Liquefied air is used to recover the majority of commercially produced nitrogen gas. The majority of that amount is utilized to make ammonia using the Haber process. Nitric acid is also produced in large quantities.
Application of Nitrogen
- For the manufacturing of chemicals and electrical compartments, nitrogen supplies a blanketing for our environment.
- In agriculture, nitrogen is utilised as a fertiliser to encourage growth.
- Nitrogen is used as a pressurized gas for oil.
- It is also used as a refrigerant for freezing things.
- It is used to make explosives.
- Treatment and protectant of metals are done in exposed Nitrogen, instead of Oxygen.
Related Post:








 ICMAI CMA Foundation Result 2025 Out, Do...
ICMAI CMA Foundation Result 2025 Out, Do...
 NEET UG 2026 Registration: NTA releases ...
NEET UG 2026 Registration: NTA releases ...
 CUET UG 2026 Online Registration Started...
CUET UG 2026 Online Registration Started...














