Table of Contents
Valency of zinc
The valency of zinc (Zn) is +2. What is Valency the Answer is The number of atoms of one element coupled with one atom of another element to form a molecule is known as valency. The term “valency” is frequently used to refer to the molecular weight of a substance. The combining power of an atom is measured by its valency. The number of electrons in an element’s outermost shell determines its valency. An element’s valency can be increased or decreased by adding or losing electrons.
The valency of an element is significant because it dictates the strength of the atom-atom bond. The stronger the relationship, the higher the valency. This is why high-valency elements are frequently used in chemical reactions because they create strong interactions with other atoms. In chemistry, valency is also significant because it helps us comprehend how atoms link together to form molecules. We can better predict how molecules will behave with each other if we understand valency. This makes it easy to develop novel chemicals for a variety of applications. Valency is a fundamental idea that aids us in comprehending our surroundings.
Valency of zinc
The valency of zinc (Zn) is typically +2.
Valency refers to the combining capacity of an element, specifically the number of electrons an atom can gain, lose, or share to form chemical bonds with other atoms. Zinc is a transition metal and belongs to Group 12 of the periodic table. It has two valence electrons in its outermost shell, which allows it to readily lose those electrons and form a positive 2+ charge. This valency of +2 is the most common for zinc in chemical reactions and compounds.
Valency of Zinc is positive and negative
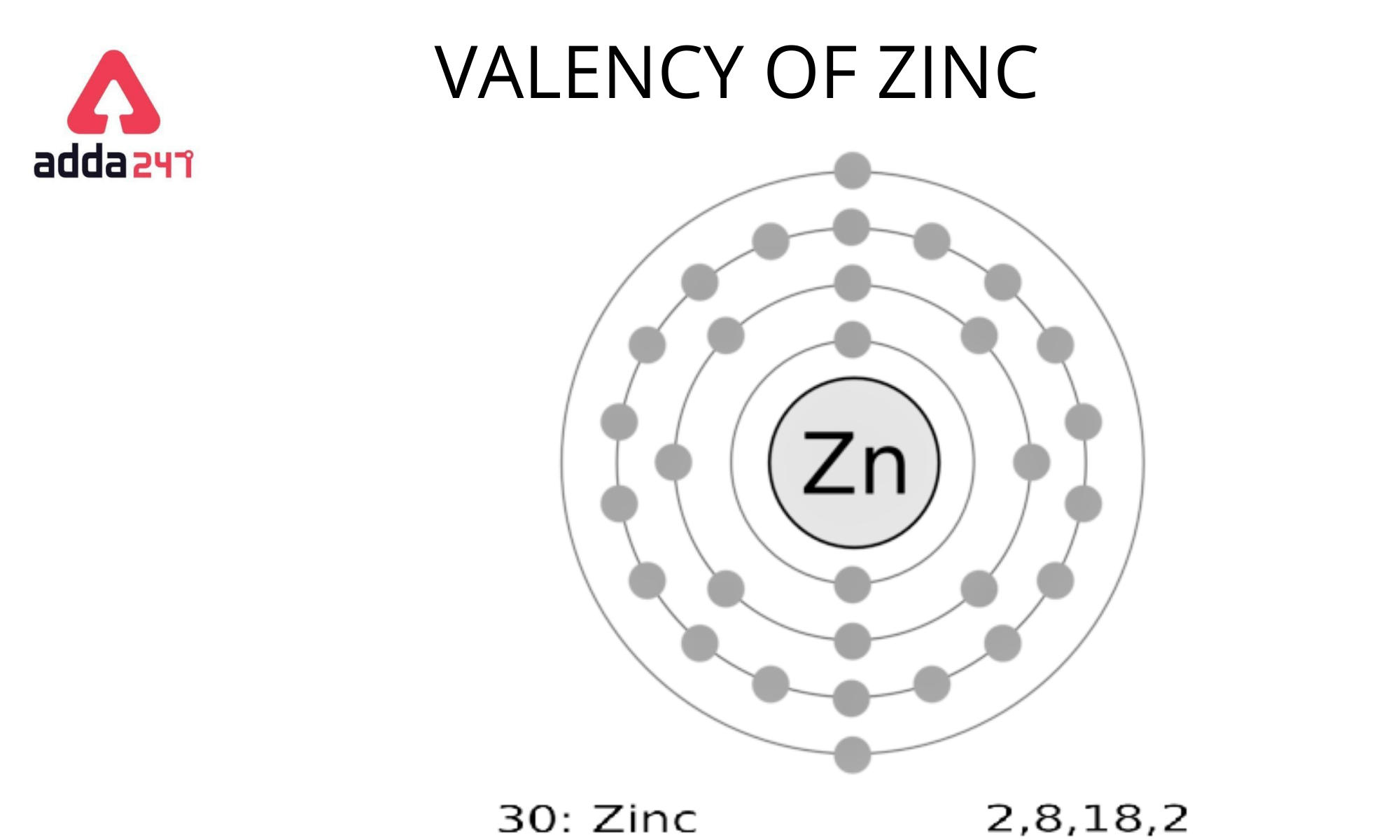
The periodic table’s 30th element is zinc.
We can figure out the number of atoms in each shell by using 2n2.
K shell: 2*(1)2 = 2, L shell: 2*(2)2 = 8, M shell: 2*(3)2 = 18
(2+8+18 = 28) => Total electrons in the first three shells
Every element now aspires to be an octet.
Zinc has a total of 30 electrons, with 28 in the first three shells and two in the fourth shell.
It must either lose two electrons or gain six to attain octet.
Because it is easier to lose two electrons in the outermost shell, Zinc loses these two electrons and creates a 2+ action.
As a result, Zinc has a valency of Zinc is plus two (2).
zn valency- valency of zn
Zn belongs to Group 12 of the periodic table and has two valence electrons in its outermost shell. Zinc tends to lose these two electrons to achieve a stable electron configuration, resulting in a valency of +2. This means that zinc typically forms compounds with a 2+ charge, where it donates its two valence electrons to bond with other elements.
Properties of Zinc
- Zinc has a bluish silver surface when it is first cast, but it progressively oxidises in the air to form a greyish protective oxide covering.
- Highly pure zinc (99.99 percent) is ductile; the so-called prime western grade (99.8% pure) is brittle when cold, but can be rolled into flexible sheets above 100 degrees Celsius (212 degrees Fahrenheit).
- Zinc crystallises in a close-packed hexagonal form.
- When iron and zinc are combined in an electrolytic cell and exposed to a corrosive solution, the zinc is attacked (oxidised to the Zn2+ ion) first due to its greater electrode potential.
Uses
- Zinc metal is primarily used to protect iron and steel from corrosion, as well as to create brasses and alloys for die-casting.
- When exposed to the atmosphere, zinc creates an impermeable layer of its oxide, making it more resistant to common atmospheres than iron and corroding at a far slower rate.
- Because zinc oxidises faster than iron, the steel surface receives some protection, even if some of it is exposed through fissures.
- Hot-dip galvanising or electrogalvanizing are both used to create the zinc coating.
Occurrence
- Zinc is somewhat more abundant than copper, accounting for 65 grammes (2.3 ounces) per tonne of Earth’s crust.
- Sulfide sphalerite (zinc blende) is the most common zinc mineral, accounting for approximately all of the world’s zinc ore, together with its oxidation products smithsonite and hemimorphite.
- Native zinc has been discovered in Australia, New Zealand, and the United States, and China, Australia, and Peru are the top zinc producers in the early twenty-first century.
- Zinc is a vital trace essential nutrient, where it is found in high concentration in red blood cells as an important component of the enzyme carbonic anhydrase, which drives numerous carbon dioxide metabolism pathways.
- The zinc found in the pancreas may help with insulin storage. Some enzymes in the gastrointestinal system that break down protein contain zinc.
- Pecan rosette, tiny leaf, and mottle leaf are diseases caused by zinc deficiency in nut-bearing and fruit trees. Zinc transports oxygen in snails’ blood via hemosycotypsin, similar to how iron transports oxygen in human blood via hemoglobin.
Related Post:
- Valency Of Carbon And Its Compounds?
- Moment Of Inertia- Definition, Formula, Factors, Example, Unit
- Magnetic Flux- Definition, Density, Formula, SI Unit
- RRB NTPC Full Form In Railways
- Kolbe Electrolysis Reaction- Definition, Mechanism Class 12
Valency of Zinc QNAs
Ques. Why valency of zinc is 2?
Ans. The valency of zinc is 2. Zinc is a metal that has an electropositive charge. It will easily lose electrons as a result of this. Zinc’s valency is two, hence it will lose two electrons.
Ques. What is the valency of zinc and oxygen?
Ans. Zinc has a valence of +2 in zinc oxide. In other words, zinc has the ability to transfer two electrons to oxygen, and oxygen gains two electrons from it, resulting in the compound zinc oxide.
Ques. What is zinc good for?
Ans. Zinc is a nutrient that is found all throughout your body and aids in the functioning of your immune system and metabolism. Zinc is also necessary for wound healing and the development of your senses of taste and smell. Your body normally obtains enough zinc from a diverse diet. Chicken, red meat, and fortified breakfast cereals are all good sources of zinc.
Ques. Is Zinc consumption required for human body?
Ans. Zinc isn’t required in huge amounts by the human body. Adults should consume 8 to 11 milligrammes per day. Although it’s usual to have slightly low zinc levels, taking a multivitamin and eating a nutritious diet should provide you with enough zinc.
Ques. What are the side effects of zinc?
Ans. Zinc may cause nausea, vomiting, diarrhoea, a metallic taste, renal and stomach damage, and other negative consequences in certain people. Zinc can produce burning, stinging, itching, and tingling when applied to injured skin. When used by mouth in levels larger than 40 mg per day, zinc is POSSIBLY SAFE.


 NEET City Intimation Slip 2025 Release D...
NEET City Intimation Slip 2025 Release D...
 CUET UG Date Sheet 2025 @cuet.nta.nic.in...
CUET UG Date Sheet 2025 @cuet.nta.nic.in...
 UP, MP, CBSE Board Result 2025 Live Upda...
UP, MP, CBSE Board Result 2025 Live Upda...










